INTRODUCTION
Stroke-associated pneumonia (SAP) is a common medical complication in acute ischemic stroke (AIS) with an incidence of 6.7–37.98%1,2. According to the Pneumonia in Stroke Consensus Group (PISCES) group, SAP is defined as a spectrum of lower respiratory tract infections that arise within 7 days following stroke onset in non-ventilated patients3. SAP has been associated with poor functional outcomes, increased treatment expenditures, length of stays, mortality risk, and a significant reduction in quality of life2,4. In order to improve patients’ clinical outcomes, it is imperative to identify high-risk individuals.
In clinical practice, the diagnosis of SAP is often overlooked which potentially results in postponed or inappropriate antibiotic treatment5. There are several clinical scoring systems that have been proposed to predict SAP6,7. Most of them rely on clinical manifestations and include numerous scoring parameters. However, the presence of atypical clinical presentations in some patients may affect the accuracy of these scoring methods, thereby complicating SAP prediction5. Moreover, radiological imaging such as chest X-ray (CXR) is not very sensitive with only 36% of suspected SAP patients having typical diagnostic findings on the initial CXR8,9. The need for early identification of SAP is crucial in AIS patient survival; therefore a more accessible and objective predictor is required.
Serum albumin, a key plasma protein synthesized by the liver, serves as a major antioxidant, accounting for a significant portion of serum’s antioxidant capacity. A decrease in serum albumin levels in hospitalized patients is linked to a number of clinical processes, including infection, which inhibits hepatic albumin synthesis by causing systemic inflammation. Additionally, inflammatory mediators, including chemokines, increase vascular permeability, leading to the extravascular loss of albumin and exacerbating hypoalbuminemia. Serum albumin levels may serve as an early biomarker of microvascular alterations and, consequently, an indirect indicator of infection severity, highlighting its potential role as a prognostic factor in infections10-12. Therefore, in this study, we aimed to synthesize current evidence of serum albumin in SAP and provide a comprehensive understanding of its potential role in clinical decision-making.
METHODS
This systematic review was designed and conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 statement guideline and registered in the PROSPERO database (CRD42024619564)13.
Eligibility criteria
This study included all previously published observational studies in English that investigated the association between serum albumin levels and SAP, with publications up to the year 2024 considered for inclusion. The diagnosis of SAP was based on the modified criteria established by the Centers for Disease Control and Prevention (CDC)8. Studies were excluded if they were review articles, case reports, case series, conference abstracts, book chapters, commentaries, editorials, or involved non-human subjects. Additionally, studies lacking full-text availability or not directly relevant to the research topic were excluded from the analysis.
Search strategy and selection process
A comprehensive literature search was conducted using the electronic databases PubMed, ProQuest, EBSCOhost, and Google Scholar. Four independent authors identified relevant studies using the following search terms: {((Stroke[MeSH Terms]) AND (Pneumonia[MeSH Terms])) OR (Stroke associated pneumonia) OR (Stroke-associated pneumonia)} AND {(Albumin[MeSH Terms]) OR (Albumins[MeSH Terms])}.
All retrieved records were imported into Zotero version 6.0.30 for reference management and subsequently screened for duplicates, titles, and abstracts14. Studies that did not meet the inclusion criteria based on title or abstract were excluded through independent review. Full texts of potentially eligible articles were then assessed by the same four authors. Any discrepancies in study selection were resolved through discussion and consensus among all authors.
Data collection process
Data extraction was conducted independently by four authors. Any discrepancies arising during the process were resolved through consensus, with input from all four authors when necessary. The extracted data included the authors’ names, year of publication, country of origin, study design, patient characteristics (sample size and age), methods of population matching, adjusted confounding variables or exclusion criteria, and the primary outcomes of interest.
Study risk of bias assessment
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS), which is applicable to cohort, case-control, and cross-sectional study designs15. The scale evaluates studies based on multiple methodological domains. Each study received an overall quality rating categorized as good, fair, or poor. Four authors independently conducted the assessments, and any discrepancies were resolved through discussion with the full review team to reach consensus.
Effect measures
Serum albumin level was reported in g/L. For normally distributed data, values were expressed as mean ± standard deviation (SD). Data originally presented as median with interquartile range (IQR) were converted to mean and SD using appropriate statistical methods to enable inclusion in the meta-analysis16. Standardized mean differences (SMDs) were employed as the effect size for continuous variables to assess the association between SAP and serum albumin levels. Corresponding p-values were reported to indicate statistical significance, with a threshold of p≤0.05 considered statistically significant.
Synthesis methods and statistical analysis
Quantitative synthesis was performed using Review Manager (RevMan; Cochrane Collaboration), version 5.4 (Copenhagen, Denmark)17. The mean difference in serum albumin levels between the SAP and non-SAP groups was calculated to facilitate analysis. Data were analyzed using totals and subtotals with corresponding 95% confidence intervals (CIs).
The selection of the random-effects model for the meta-analysis was guided by the diversity of analytical and assessment methods across the included studies. This model accounts for variability between studies by assuming that the true effect size may differ across populations, thereby providing more balanced weighting among studies. Additionally, the random-effects model enhances the generalizability of findings to broader populations, including those represented in future research. Pooled effect estimates for continuous outcomes were calculated using the inverse variance method. SMDs were employed as the preferred effect size metric for continuous data.
Publication bias was assessed using funnel plot analysis, in which the effect size of each included study was plotted against the inverse of its standard error (SE). To evaluate heterogeneity among studies, the I² statistic was calculated with values interpreted as follows: <25% minimal, 25–50% low, 50–75% moderate, and >75% high heterogeneity. In cases where significant heterogeneity was observed, potential sources were explored through sensitivity analyses to assess the robustness and reliability of the findings.
RESULTS
Literature search
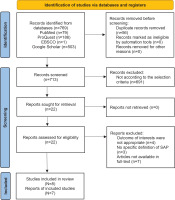
The study selection process and corresponding results are outlined in the flow diagram presented in Figure 1. Initially, 769 records were identified through the systematic search strategy. Following the removal of duplicates, 713 studies remained. These were screened in accordance with the predefined eligibility criteria. Of these, 691 were excluded based on title and abstract screening for irrelevance, and an additional 14 studies were excluded after full-text assessment due to non-fulfillment of inclusion criteria.
Ultimately, 8 studies met the criteria for inclusion in the systematic review, with 7 providing sufficient data for inclusion in the meta-analysis. Despite a comprehensive search, no unpublished studies meeting the eligibility criteria were identified. This absence is unlikely to have influenced the overall conclusions and may reduce the risk of qualitative publication bias.
Characteristics of the included studies
The characteristics of the 8 included studies1,2,5,18–22 are reported in Table 1. All the studies included in this review were cohort studies conducted in China. The mean ages of SAP and non-SAP populations ranged from 70.49 ± 11.08 to 78.30 ± 7.53 years, and 64.47 ± 11.91 to 68.65 ± 12.63 years, respectively.
Table 1
Study characteristics
| Authors Year Country | Type of study | Population | Type of stroke and diagnosis criteria | Population matching | Exclusion criteria for eligibility | Outcome of interest | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Age (years) | ||||||||
| SAP | Non-SAP | SAP | Non-SAP | ||||||
| Li et al.2 2023 China | Cohort | 184 Male: 119 (64.7%) Female: 65 (35.3%) | 1359 Male: 866 (63.7%) Female: 493 (36.3%) | 70.49 ± 11.08 | 64.47 ± 11.91 | AIS was confirmed by DWI-MRI according to the WHO criteria | Age, sex, risk factors (drinking, smoking, atrial fibrillation, and hypertension), stroke severity (NIHSS score on admission ≤4 or >4), and treatments (intravenous thrombolysis, endovascular thrombectomy, conservative treatment) | 1) Pulmonary edema, pulmonary embolism, pulmonary atelectasis, tuberculosis, pulmonary tumor, or noninfectious interstitial lung disease; and 2) pneumonia prior to admission | Identify the best predictive index and its correlation with SAP |
| Huang et al.5 2022 China | Cohort | 92 Male: 42 (45.7%) Female: 50 (54.3%) | 674 Male: 369 (54.7%) Female: 305 (45.3%) | 78.30 ± 7.53 | 68.65 ± 12.63 | AIS was confirmed by CT or MRI | A2DS2 score, first symptom to admission time, thrombolytic therapy, use of nasogastric tube, smoking history, drinking history, WBC count, neutrophil count, lymphocyte count, and hemoglobin concentration | 1) Pneumonia before stroke; 2) active infection within 2 weeks of admission or prophylactic antibacterial therapy; 3) dysphagia before stroke; 4) severe hepatic or renal diseases; 5) hematological disease or cancer; 6) received immunosuppressant treatment; 7) major trauma or surgery; and 8) gastrointestinal bleeding present | Identify the association between the baseline hypersensitive C-reactive protein-albumin ratio (CAR) and stroke-associated pneumonia (SAP) during hospitalization and the short-term prognosis in patients with acute ischemic stroke (AIS) |
| Chen et al.18 2022 China | Cohort | 897 Male: 605 (67.45%) Female: 292 (32.55%) | 4276 Male: 2678 (62.63%) Female: 1598 (37.37%) | 72.26 ± 10.95 | 66.83 ± 11.68 | AIS was confirmed by CT or MRI | Age, gender, and NIHSS | 1) Fever or active infection within 2 weeks before admission; and 2) administered with albumin during hospitalization | Identify the association between A/G and SAP, helping physicians identify those AIS patients at high SAP risk and providing more accurate guidance |
| Lin et al.1 2022 China | Cohort | 100 Male: 63 (63.0%) Female: 37 (37%) | 832 Male: 533 (64.1%) Female: 299 (35.9%) | 71.47 ± 13.16 | 66.00 ± 10.40 | AIS was confirmed by CT or MRI | Age, current smoking, drinking, NIHSS, previous stroke, diabetes mellitus, AF, thrombolysis, dysphagia, aPTT, D-dimer, PLT, length of hospital stay, and discharge mRS score | 1) TIA; 2) active infection within 2 weeks before admission or prophylactic use of antibiotics; 3) a history of CNS diseases such as brain trauma, cerebral hemorrhage, or hydrocephalus; 4) severe liver disease; and 5) severe kidney disease | Identify the relationship between SAP and FAR |
| Yan et al.19 2021 China | Cohort | 417 Male: 275 (65.95%) Female: 142 (34.05%) | 2756 Male: 1695 (61.5%) Female: 1061 (38.5%) | 71.77 ± 11.69 | 67.39 ± 11.51 | AIS was confirmed by CT or MRI | Age, NIHSS score, dysphagia, length of hospitalization, atrial fibrillation, and leukocyte | 1) Fever or infectious diseases within two weeks before admission; 2) severe diseases including cancer, heart failure, renal insufficiency, acute MI or liver dysfunction; 3) pneumonia before stroke; 4) TIA; and 5) administered with albumin during hospitalization | Identify the role of LAR in predicting the incidence of SAP in AIS patients |
| Wang et al.21 2024 China | Cohort | 85 Male: 56 (65.88%) Female: 29 (34.12%) | 371 Male: 224 (60.38%) Female: 147 (39.62%) | 71.24 ± 17.35 | 66.95 ± 12.65 | Stroke was confirmed by CT or MRI | N/A | 1) Pre-existing pneumonia prior to admission; and 2) a history of hematological diseases, malignancy, immunosuppressant treatment, or severe hepatic or renal dysfunction | Developed an ANN model to predict the risk of SAP in stroke patients and compared its predictive performance with that of a traditional LR model |
| Song et al.22 2022 China | Cohort | 80 Male: 49 (61.3%) Female: 31 (38.7%) | 807 Male: 540 (66.9%) Female: 267 (33.1%) | 71.3 ± 12.2 | 66.7 ± 11.8 | AIS was confirmed by the WHO criteria | N/A | 1) age < 18 years; 2) TIA or cerebral hemorrhage; 3) active pulmonary or other sites infection within the last 14 days; 4) history of severe hepatic diseases, hematological malignancy, or immunosuppressant treatment; 5) recent history of major trauma or surgery | Evaluate the relevance of the A2DS2 score with SAP in AIS patients with T2DM and establish an individualized nomogram for discerning SAP in these patients by combining the A2DS2 score with simple nutrition-related biomarkers |
| Dai et al.20 2022 China | Cohort | 424 Male: 283 (66.75%) Female: 141 (33.25%) | 2,992 Male: 1925 (64.34%) Female: 1067 (35.66%) | 71.83 ± 11.04 | 65.89 ± 11.46 | AIS was confirmed by MRI | Age, atrial fibrillation, NIHSS scores, length of hospitalization, leukocyte, dysphagia, and treatment method | 1) Suffered from fever or infection diseases within half a month before admission; and 2) usage of albumin during hospitalization | Identify the role of GNRI (Geriatric Nutritional Risk Index) in SAP |
[i] CAR: C-reactive protein-albumin ratio. SAP: stroke-associated pneumonia. AIS: acute ischemic stroke. GNRI: Geriatric Nutritional Risk Index. NIHSS: National Institutes of Health Stroke Scale. CT: computed tomography. MRI: magnetic resonance imaging. T2DM: type 2 diabetes mellitus. TIA: transient ischemic attack. A2DS2 score: age, atrial fibrillation, dysphagia, sex, stroke severity score. CNS: central nervous system.
All studies used the modified Centers for Disease Control and Prevention (CDC) criteria as diagnosis criteria for SAP8. Most studies specifically examine SAP events in ischemic stroke, except for Wang et al.21 who did not specify the type of stroke included in their study.
Quality assessment
The quality of the selected studies was evaluated using the NOS for cohort studies. Based on the assessment, six studies were classified as high quality1,2,5,18–20, while two were rated as poor quality due to a lack of comparability between study groups21,22 (Table 2).
Table 2
Bias assessment risk for cohort studies
| Authors Year | Selection | Comparability | Outcome | Conclusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | C1 | O1 | O2 | O3 | ||
| Li et al.2 2023 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | - | Good |
| Huang et al.5 2022 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | Good |
| Chen et al.18 2022 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | - | Good |
| Lin et al.1 2022 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | - | Good |
| Yan et al.19 2021 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | - | Good |
| Wang et al.21 2024 | ☆ | ☆ | ☆ | ☆ | - | ☆ | ☆ | - | Poor |
| Song et al.22 2022 | ☆ | ☆ | ☆ | ☆ | - | ☆ | ☆ | - | Poor |
| Dai et al.20 2022 | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | - | Good |
Meta-analysis results
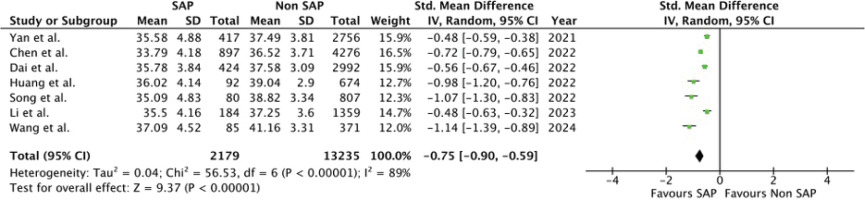
A total of 7 studies2,5,18–22 were included in the meta-analysis (Table 3). All studies reported significantly lower serum albumin levels in the stroke-associated pneumonia (SAP) group compared to the non-SAP group (p<0.001). One additional study by Lin et al.1 could not be incorporated into the meta-analysis due to data format limitations; however, it reported median serum albumin levels of 36.45 g/L in the SAP group and 38.1 g/L in the non-SAP group, supporting the overall trend.
Table 3
Results of studies included in the meta-analysis
| Authors Year | Albumin Mean ± SD | ||
|---|---|---|---|
| SAP | Non-SAP | p | |
| Li et al.2 2023 | 35.4987 ± 4.16058 | 37.2469 ± 3.56608 | <0.001 |
| Huang et al.5 2022 | 36.0197 ± 4.1424 | 39.035 ± 2.8973 | <0.001 |
| Chen et al.18 2022 | 33.79 ± 4.18 | 36.52 ± 3.71 | <0.001 |
| Yan et al.19 2021 | 35.58 ± 4.88 | 37.49 ± 3.81 | <0.001 |
| Wang et al.21 2024 | 37.0886 ± 4.5249 | 41.1577 ± 3.3118 | <0.001 |
| Song et al.22 2022 | 35.0885 ± 4.8318 | 38.8249 ± 3.3419 | <0.001 |
| Dai et al.20 2022 | 35.78 ± 3.84 | 37.58 ± 3.09 | <0.001 |
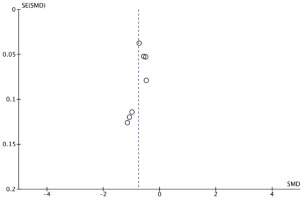
The pooled analysis, illustrated in Figure 2, demonstrated a significant association between SAP and reduced serum albumin levels (SMD= -0.75; 95% CI: -0.90 – -0.59); p<0.00001). Substantial heterogeneity was observed among the included studies (I²=89%), necessitating the use of a random-effects model. The funnel plot (Figure 3) appears symmetrical, suggesting no evidence of publication bias.
DISCUSSION
SAP significantly worsens clinical outcomes, increasing mortality rates and severely compromising quality of life in stroke patients2,4. Early identification of individuals at high risk for SAP is critical for guiding personalized interventions and reducing the burden of this serious complication. To the best of our knowledge, this is the first meta-analysis evaluating serum albumin levels for predicting SAP. The finding of our study is that SAP patients had a statistically significant lower serum albumin level than non-SAP patients (SMD= -0.75; 95% CI: -0.90 – -0.59; p<0.00001).
Albumin is readily accessible and has shown inverse association with pneumonia occurrence19. Moreover, albumin levels can decrease swiftly during acute infections, often within hours, even in patients with adequate nutritional status, likely due to increased capillary filtration and the associated loss of albumin from the vascular space23. Several mechanisms may account for the observed association between serum albumin levels and SAP. The first possibility is the role of albumin in modulating the immune system. Hypoalbuminemia is often indicative of poor nutritional status and compromised immune function, both of which may predispose individuals to an increased risk of pneumonia18,19,24. A second potential mechanism involves the neuroprotective properties of albumin. Elevated albumin levels may reduce the risk of SAP indirectly by mitigating stroke severity. Albumin possesses potent antioxidant and anti-inflammatory properties, which contribute to neuroprotection in the context of acute cerebrovascular events19,24,25. Furthermore, albumin may aid in restoring vascular patency post-thrombolysis by counteracting blood stagnation and thrombosis. Its ability to lower hemoglobin concentration and erythrocyte sedimentation rate may also support improved reperfusion19,26. A third explanation centers on the capacity of albumin to influence inflammatory processes. Albumin can modulate endotoxin-induced inflammation through its binding affinity to lipid A, reactive oxygen species, lipopolysaccharides, and other bacterial components such as lipoteichoic acid and peptidoglycan. Moreover, albumin contributes to plasma volume expansion, which may play a role in the regulation of systemic inflammatory responses19,27,28. Overall, albumin is a key factor in inflammatory modulation and is closely associated with the pathogenesis of pneumonia. Additionally, as an indicator of nutritional status, albumin reflects the overall health of patients and is linked to the risk of post-stroke complications, especially SAP19,29.
The majority of the studies included in this review indicated a higher prevalence of SAP in male patients compared to females1,2,18-22, with the exception of a study by Huang et al.5, although this finding was not statistically significant. These findings are consistent with a meta-analysis by Ahmad et al.30, which identified a higher susceptibility to post-stroke pneumonia in male patients (OR=1.09; 95% CI: 1.04–1.15, p=0.001). This gender disparity may reflect true differences in incidence rates between males and females, potentially linked to the greater prevalence of current and former smoking behaviors among males30. Notably, the gap in susceptibility appears to diminish after menopause, suggesting a potential neuroprotective role of endogenous estrogens in mitigating stroke-related complications31. All of the studies included in this review demonstrated a higher prevalence of SAP in older age groups. These results are consistent with a meta-analysis by Ahmad et al.30, which identified advanced age as a significant risk factor (OR=5.24; 95% CI: 4.24–6.18, p=0.001). These findings can be attributed to the immune system deficiencies commonly observed in older patients, which increase their susceptibility to infections32.
Heterogeneity and publication bias analysis
The I² statistic for heterogeneity was 89%, indicating a high level of heterogeneity among the included studies. This heterogeneity can be attributed to variations across clinical, methodological, and statistical domains. Clinically, differences in participant characteristics and outcome measures likely contributed to the observed variability. The sample sizes across studies ranged from 456 to 5173 participants, and such disparities in population size may influence the likelihood and magnitude of effect sizes. Methodologically, substantial heterogeneity may have arisen from differences in study design and approaches to population matching. While all studies excluded individuals with pre-existing pneumonia prior to stroke onset or hospital admission, inconsistencies in methodological quality persisted. Two studies were rated as poor quality due to the absence of comparability, whereas the remaining studies were assessed as being of good quality. These variations in risk of bias may have further contributed to heterogeneity. Statistically, inconsistency in data reporting formats added to the variation: three studies reported outcomes using median and interquartile range, while four reported mean and standard deviation. Nevertheless, all data were synthesized using SMD in the meta-analysis, which served to mitigate some degree of statistical heterogeneity.
Strengths and limitations
This systematic review has several strengths. To our knowledge, this is the first meta-analysis to examine the potential role of albumin as a predictive biomarker for SAP. The majority of the included studies were of good quality, thereby enhancing the credibility of the findings. Additionally, the review incorporates a large aggregated sample size, which contributes to increased statistical power and robustness of the results. However, certain limitations should be acknowledged. All included studies were conducted within Chinese patient populations, which may introduce publication bias and potentially compromise the validity and generalizability of the findings presented in this systematic review.
Future research
Given the significant differences in serum albumin levels observed between patients with SAP and those without, future research should involve larger, more diverse cohorts to enhance the generalizability of the findings. To strengthen the current findings, a meta-analysis evaluating the predictive accuracy of albumin for the occurrence of SAP, based on sensitivity and specificity metrics should be carried out. Moreover, it is advisable for clinicians to routinely evaluate serum albumin levels in stroke patients as part of early risk stratification for SAP and potential mortality.