INTRODUCTION
Nocardia spp. are aerobic, gram-positive, filamentous bacteria commonly found in soil and decaying organic matter1. Of the more than 90 identified species, certain strains such as N. cyriacigeorgica, N. farcinica, and the N. nova complex are recognized human pathogens2. Nocardiosis typically affects immunocompromised individuals but may also occur in patients with chronic lung diseases like chronic obstructive pulmonary disease (COPD)3,4. The pathogen evades innate immunity by surviving within phagocytes through inhibition of phagosome-lysosome fusion and neutralization of reactive oxygen species4, making cell-mediated immunity essential. Risk factors include corticosteroid use, immunosuppression, and structural lung damage3,5. Pulmonary nocardiosis presents with non-specific symptoms such as cough, dyspnea, fever, and weight loss, and radiologically may mimic tuberculosis, malignancy, or fungal infections6, often delaying diagnosis. Without timely identification and treatment, the infection may disseminate, particularly to the central nerve system (CNS), leading to significant morbidity and mortality7,8.This report presents a case of Nocardia lung infection in a patient with severe COPD on long-term oxygen therapy (LTOT) and non-invasive ventilation (NIV), highlighting the importance of considering nocardiosis in patients with non-resolving pulmonary infections despite standard antimicrobial treatment.
CASE PRESENTATION
An 83-year-old male with a history of COPD, heart failure with preserved ejection fraction, on LTOT (2.5 L/min) and nocturnal bilevel positive airway pressure, presented to the emergency department with worsening dyspnea over 24 hours, productive mucopurulent cough for 20 days, and home-recorded fever up to 38.4°C. His maintenance treatment included a combination of inhaled long-acting beta-agonist (LABA), long-acting muscarinic antagonist (LAMA), and inhaled corticosteroid (ICS) via a valved holding chamber, along with loop diuretics, antihypertensives, and a sodium-glucose co-transporter 2 (SGLT2) inhibitor. He reported 3–5 COPD exacerbations annually, with 1–2 requiring hospitalization, and had received a short course of oral corticosteroids a few months earlier for a home-treated exacerbation. Inhaled corticosteroids were used chronically, while oral corticosteroids were prescribed only during exacerbations. He had recently completed a 10-day course of oral levofloxacin without improvement.
On examination, he was tachypneic and in mild respiratory distress but hemodynamically stable. Bilateral crackles were present on auscultation. Arterial blood gas analysis revealed compensated hypercapnic respiratory failure (pH 7.45, pCO2 62 mmHg, pO2 45 mmHg, HCO3- 28 mmol/L, SpO2 91% on 2.5 L/min oxygen). Laboratory tests showed leukocytosis and elevated inflammatory markers. A chest X-ray (Figure 1) demonstrated bilateral ground-glass opacities and signs of emphysema, interpreted as pneumonia superimposed on COPD.
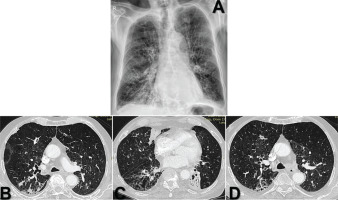
Figure 1
Initial imaging findings at admission: A) Chest X-ray shows bilateral ground-glass opacities and underlying emphysematous changes; B–D) High-resolution computed tomography (HRCT) reveals scattered nodular opacities (up to 16 mm) predominantly in the right upper and middle lobes, tree-in-bud and centrilobular nodules primarily in the left upper lobe and extensive emphysematous and bronchiectatic changes with mild pleural effusions
Rapid antigen tests for SARS-CoV-2 and common respiratory viruses were negative. The patient was admitted with a working diagnosis of acute exacerbation of COPD and started on nebulized bronchodilators (ipratropium bromide), intravenous 40 mg of methylprednisolone, and empirical broad-spectrum antibiotics (piperacillin/tazobactam and azithromycin).
Despite therapy, after 5 days the patient remained febrile and dyspneic. A high-resolution computed tomography (HRCT) scan (Figure 1) showed scattered nodular opacities (up to 16 mm) predominantly in the right upper and middle lobes; tree-in-bud and centrilobular nodules mainly in the left upper lobe; mild bilateral pleural effusions; adjacent atelectasis; and extensive upper lobe-predominant emphysematous and bronchiectatic changes.
Therefore, due to lack of clinical improvement and atypical HRCT findings, sputum cultures were sent. Gram stain and culture (Figure 2) revealed abundant Nocardia spp. The upper lobe-predominant distribution, tree-in-bud pattern, subacute course, and poor response to standard antibiotics were consistent with the diagnosis of pulmonary nocardiosis.
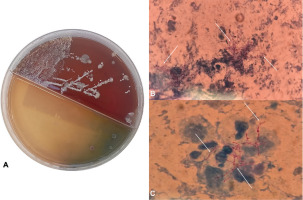
Figure 2
Microbiological identification of Nocardia spp: A) Colony growth of Nocardia spp. on selective medium in a Petri dish after incubation; B–C) Microscopic visualization of Nocardia spp. on Gram stain, showing filamentous, branching structures (marked with arrows) consistent with Nocardia morphology
Blood cultures were negative, and the MRI of the brain showed no signs of CNS involvement. HIV serology was negative, and immunoglobulin levels were within normal limits. The patient had no known immunosuppressive condition but had frequent steroid use and low body mass index. Targeted antimicrobial therapy with intravenous trimethoprim-sulfamethoxazole and imipenem/cilastatin was initiated. Although Nocardia was the only organism isolated, imipenem/cilastatin was concurrently selected to maintain empirical coverage for Pseudomonas aeruginosa, given the patient’s severe COPD exacerbation, high risk for pseudomonal infection, and the possibility of false-negative sputum cultures. As imipenem/cilastatin also offers effective activity against Nocardia spp., it served as a suitable dual-purpose agent in this clinical context. The patient showed marked clinical improvement over the following days, with resolution of fever, normalization of inflammatory markers, and improved respiratory symptoms.
After three weeks of inpatient therapy, he was discharged afebrile and clinically stable. He continued oral trimethoprim-sulfamethoxazole for outpatient therapy. At follow-up three weeks post-discharge (six weeks from admission), the patient remained asymptomatic and repeat chest CT (Figure 3) showed significant radiographic improvement. He expressed relief and satisfaction with his recovery, emphasizing his appreciation for finally receiving a diagnosis after weeks of persistent symptoms. Prolonged oral therapy was advised for at least three months, with continued monitoring.
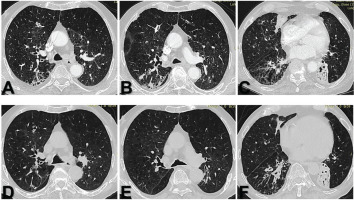
Figure 3
Follow-up HRCT imaging three weeks post-discharge: A–C) Follow up HRCT images showing resolution of nodular opacities and tree-in-bud pattern with persistent background emphysema and bronchiectasis; D–F) Corresponding baseline HRCT images for comparison, demonstrating the previously noted abnormalities before treatment
DISCUSSION
This case underscores the need to consider Nocardia as a potential pathogen in COPD patients, especially those with frequent exacerbations, prolonged corticosteroid use, and on long-term non-invasive ventilation. In this patient, the subacute presentation, lack of response to conventional antibiotics, and upper lobe-predominant imaging findings were key clues prompting further investigation. Sputum culture confirmed pulmonary nocardiosis, allowing timely initiation of targeted therapy and clinical resolution. Given the overlapping features of pulmonary nocardiosis with common bacterial and mycobacterial infections, a high index of suspicion is essential. Early microbiological confirmation can facilitate appropriate management and improve outcomes in this vulnerable patient population.
Strengths and limitations
One strength of this case is the detailed clinical and radiological documentation in a high-risk COPD patient without known immunodeficiency, highlighting an atypical but important differential diagnosis. While this case adds to the growing recognition of Nocardia as an underdiagnosed pathogen in COPD, it represents a single case and, therefore, may limit the generalizability of its findings. However, its clinical presentation and imaging features are consistent with those described in previous studies, including cases involving immunocompetent COPD patients3-5. Prior analyses have also emphasized the diagnostic challenge posed by the radiological overlap with other pulmonary diagnoses6, further supporting the importance of maintaining a high index of suspicion in similar scenarios.
CONCLUSION
Nocardia spp. are capable of intracellular survival by evading host immune mechanisms, primarily through the inhibition of phagosome-lysosome fusion and the neutralization of reactive oxygen species4. These virulence traits allow the pathogen to persist in the lungs and contribute to its opportunistic nature in hosts with compromised immune defenses3-5. Radiologically, pulmonary nocardiosis can resemble tuberculosis, fungal infections, or malignancy, often presenting with nodular opacities and tree-in-bud patterns6. These non-specific findings frequently lead to diagnostic delays, underscoring the importance of clinical suspicion and targeted microbiological testing when patients do not respond to conventional therapies.


