INTRODUCTION
With an increasing aging population, lower respiratory tract infections (LRTIs) are the deadliest infectious diseases worldwide with high morbidity and mortality, especially in elderly and immunocompromised populations1,2. The early and accurate determination of infectious pathogens in LRTIs is very difficult, especially in grassroots hospitals, as LRTIs are caused by hundreds of pathogens, including bacteria, virus, fungi, chlamydia and mycoplasma. In addition, for some immunocompromised patients, almost all bacteria or fungi can be considered potential pathogens in LRTIs3. Currently, conventional microbiological test methods (CMTs) including microscopy, pathogen culture and isolation, biochemical testing, enzyme-linked immunosorbent assay (ELSA), and polymerase chain reaction (PCR) testing, are applied to identify LRTIs pathogens. However, these methods have shortcomings in terms of sensitivity, specificity, timeliness, and amount of information obtained and the shortcomings of these detection methods are particularly evident in grassroots hospitals4. Due to the lack of microbiological diagnostic methods, empirical use of antibiotics may exacerbate re-infection and lead to the emergence of antibiotic resistance and multidrug-resistant pathogens5. Given the above reasons, metagenomic next-generation sequencing (m-NGS) with a broad detection spectrum, including bacteria, viruses, fungi, atypical pathogens, parasites, and even new microorganisms, the diagnostic efficiency of m-NGS is hardly affected by antibiotics6. Therefore, m-NGS has been applied in clinical settings such as the diagnosis of infectious diseases and the acquisition of novel microbial genome sequences7, emerging as a promising single, universal pathogen detection method for infectious disease diagnosis and tracking. However, the high economic cost of m-NGS detection and the limitations of national medical insurance policies (NMIP) have a bleak prospect for its development in grassroots hospitals. Multiple PCR-based targeted NGS (t-NGS) is performed by making a panel of the specific sequences of prescreened pathogens, amplifying the target genes, obtaining information about the enriched nucleic acids through a high-throughput sequencing platform, and then analyzing the results by bioinformatics to identify pathogens. Compared with PCR technology and NGS technology, t-NGS technology is more comprehensive and takes into account the advantages of both8,9. At the same time, the economic cost of t-NGS is only one-fourth of m-NGS, this may make t-NGS to be vigorously promoted and carried out in grassroots hospitals. Therefore, in solving the dilemma of LRTIs pathogen diagnosis in primary hospitals, t-NGS may demonstrate great potential, but its clinical practicality needs further in-depth research. In this study, we explored the reliability and clinical practical value of t-NGS for diagnosing pathogens of LRTIs in enrolled 140-case patients willing to undergo BALF and to compare its efficiency and concordance against CMTs.
METHODS
Study population and data collection
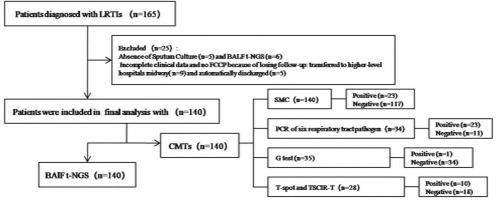
This retrospective observational study enrolled 165-case patients with suspected LRTIs who were hospitalized at the Longquan City People’s Hospital affiliated with Lishui University between 1 January 2021 and 31 March 2024. Among enrolled and confirmed patients with LRTI, including acute bronchitis (AB), community acquired pneumonia (CAP)/pneumonia (P), and acute exacerbation of chronic obstructive pulmonary disease (AECOPD), the diagnostic criteria for LRTIs are described in the relevant literature10-12. Among them, 11 cases were excluded due to lack of sputum culture (5 cases) or failure to undergo bronchoalveolar lavage fluid (BALF) testing (6 cases). And 14-case patients with incomplete clinical data and no clinical confirmed pathogens were also excluded because of losing follow-up, including 9-case patients transferred to higher-level hospitals midway and 5-case patients automatically discharged. Finally, 140-case patients with LRTIs were selected (Figure 1) and the following information of enrolled patients were recorded: demographic data, underlying medical conditions including different underlying diseases, different infectious pathogens, laboratory test results including CMTs based on the PCR of six respiratory tract pathogens including Influenza virus, Respiratory syncytial virus, Adenovirus, Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila, G test (serum 1, 3-beta-D-glucan level) for fungi, as well as T-spot and tuberculosis specific cellular immune response (TSCIR) test for Mycobacterium tuberculosis (TB), and the results of t-NGS of 140-case BALF of different sample collection times (SCTs) including on the day of admission or 3 days after admission. In addition, the replacement of antibiotics after 3 days of admission was also included in the collected data. For the convenience of research, we have included two pathogen diagnostic concepts: ‘primary clinically confirmed pathogen (PCCP)’ and ‘final clinically confirmed pathogen (FCCP)’. The former refers to the initial pathogen diagnosis of the patient upon admission (within 3 days of admission), while the latter refers to the final confirmed pathogen diagnosis of the patient upon discharge. The confirmed procedure of every patient with FCCP requires a comprehensive analysis of clinical dates including signs, symptoms, imaging features, treatment effects and the test results of CMTs and t-NGS by 2–3 senior specialist physicians, even multidisciplinary consultations involving experts in testing, microbiology, imaging, and pharmacy when necessary. Ethics approval was obtained from the ethics committee of Longquan City People’s Hospital affiliated with Lishui University (Approval number: IRB-LPHALU20220128). The necessity of informed consent was waived by the ethics committee of Longquan People’s Hospital because of the retrospective and observational nature of the research.
Sample collection
BALF or sputum samples were collected from enrolled 140-case patients and stored in sterile screw-capped cryovials. It is key to note that the selection of BALF samples were sourced from the middle segment, with the anterior segment’s collected liquid discarded. Similarly, preserved sputum samples were procured from the first deep cough of patients’ episodes in the early morning, following mouth rinsing with sterile saline 2–3 times. These samples were then transported to the designated laboratory for t-NGS within 24 h, at ≤ -20℃ to ensure sample integrity. For BALF samples, a volume of 5–10 mL was collected from each patient, while 4 mL of sputum was collected for each patient as well. These samples were carefully handled and preserved to maintain the quality of the genetic material for subsequent t-NGS analysis and CMTs test.
Targeted next-generation sequencing
The collected BALF samples from 140-case patients with LRTIs were divided into batches and sent to the same testing institution for t-NGS testing according to the corresponding t-NGS testing process of the testing agency, including sample preparation and nucleic acid extraction, library construction and sequencing, and bio-informatics analysis. The above testing workflow has been completed by Hangzhou KingMed Diagnostics. In addition, the detection scope of t-NGS has been set up into four categories by Hangzhou KingMed Diagnostics, including bacteria, viruses, fungi, and other special pathogens, totaling 151 targeted pathogens.
Interpretation of t-NGS results
Based on the experimental principle of targeted amplification of microbial sequences using specific primers, the coverage of amplicons and the normalized read count of detected microorganisms in the sample constitute the main explanatory indicators. According to the reporting guidelines of a testing institution called KingMed Diagnostics (Hangzhou), microorganisms are classified as potential pathogens and the following evaluation criteria are established. Firstly, the number of sequences containing microorganisms per 100K raw sequences is evaluated, that is, the number of homogenized sequences, where the more homogenized sequences there are, the higher the certainty of microbial content in the sample. Secondly, bioinformatics methods are used to calculate the microbial content in the sample, i.e. the estimated microbial concentration (copy/mL), but this result is not an absolute quantification and is only for clinical reference. Thirdly, the pathogenicity classification was as follows:
Class A: In respiratory specimens, it is a specific pathogenic pathogen or a clinically common pathogenic pathogen.
Class B: Opportunistic (conditional) pathogenic pathogen in respiratory specimens, with systemic or local immune deficiency/damage/deficiency, respiratory barrier dysfunction, or downward respiratory distress in patients.
Class C: It is a normal microbial community in the respiratory tract that generally does not cause infection, but there is a possibility of aspiration leading to lung abscess.
The specific explanation of the t-NGS mentioned above can be found in the Supplementary file Figure 1. Finally, two experienced senior clinicians independently conducted a comprehensive assessment of the patient’s clinical data to determine the presence of pulmonary infection and the clinical relevance of potential pathogens. However, the FCCP depends on clinical comprehensive evaluation based on the clinical characteristics of LRTIs patients including the patient’s medical history, clinical symptoms, imaging findings, t-NGS results, and CMT outcomes.
Statistical analysis
Continuous variables are described by the mean and standard deviation (SD). Categorical variables are described by frequencies and percentages and compared using the chi-squared test and Fisher’s exact test. The Agreement Test between two different methods for detecting pathogens and the FCCP was conducted using qualitative data consistency test, kappa coefficient to show significance, and the comparison of accuracy rate of FCCP (%) by Pearson chi-squared test. Statistical analysis was performed with SPSS software (version 19.0, Chicago, IL, USA). A p<0.05 was considered statistically significant. The comparison and analysis of the characteristics of pathogens detected by t-NGS for LRTIs in different age groups, seasons, merged underlying diseases, different disease types, and the comparison of positive detection rate (%) and consistency rate (%) of two detection methods for different pathogens were completed and displayed by different distribution maps made by Graphboard Template Chooser of SPSS software.
RESULTS
Epidemiological characteristics of 140 patients with LRTIs
Of 140-case enrolled LRTI patients, the mean age was 63.9 ± 16.1 (years), among them the proportion of patients aged 60–80 years was as high as 53.6%. The highest proportion of males was 68.1%, and among different types of LTRTIs, AB accounted for 16.4%, CAP/pneumonia 64.3%, and AECOPD 19.3%. In addition, the proportion of patients with ≥2 underlying diseases among patients with merged underlying diseases was as high as 33.6%, and the proportion of patients with LRTIs was almost the same in different seasons. Among the single pathogen infections detected by CMT and t-NGS testing, bacterial infections had the highest proportion (17.9%); however, fungi infections showed the lowest proportion of 0.71% (1/140). The vast majority of patients with LRTIs showed co-infected pathogens, among which bacterial, viral and fungal co-infection was the most common, accounting for 25.7%. The pre-admission exposure rate of antibiotics was as high as 62.1%. BAFL samples were collected from 140-case patients on the day of admission (82 cases) and 3 days after admission (58 cases). The main demographic and clinical characteristics of the study populations are detailed in Table 1.
Table 1
Summary of clinically relevant information of selected LRTIs patients (N=140)
| Characteristics | Patients n (%) |
|---|---|
| Age (years), mean ± SD | 63.93 ± 16.05 |
| Age (years) | |
| 18–40 | 11 (7.90) |
| 40–60 | 35 (25.00) |
| 60–80 | 75 (53.60) |
| >80 | 19 (13.60) |
| Sex | |
| Female | 44 (31.20) |
| Male | 96 (68.10) |
| LRTIs types | |
| AB | 23 (16.40) |
| CAP/pneumonia | 90 (64.30) |
| AECOPD | 27 (19.30) |
| Underlying diseases | |
| Hypertension | 17 (12.10) |
| CHD | 7 (5.00) |
| DM | 15 (10.70) |
| MT/AD | 12 (8.60) |
| CKD | 3 (12.10) |
| CLD | 1 (0.70) |
| ≥2 underlying diseases | 47 (33.60) |
| Different seasons | |
| Spring | 33 (23.60) |
| Summer | 34 (24.30) |
| Autumn | 34 (24.30) |
| Winter | 39 (27.90) |
| Pathogens by t-NGSa | |
| None | 3 (2.14) |
| Bacterial | 81 (57.86) |
| Viruses | 63 (45.00) |
| Fungi | 62 (44.29) |
| Atypical pathogens (mycoplasma or chlamydia) | 15 (10.71) |
| Single pathogens by t-NGS and CMTsb | |
| None | 3 (2.10) |
| Bacteria | 25 (17.90) |
| Viruses | 8 (5.70) |
| Fungi | 1 (0.70) |
| Atypical pathogens (Mycoplasma + Chlamydia) | 8 (5.71) |
| Co-infected pathogens by t-NGS and CMTsb | |
| Bacteria + viruses | 31 (22.10) |
| Bacteria + fungi | 3 (2.10) |
| Viruses + fungi | 14 (10.0) |
| Bacteria + viruses + fungi | 36 (25.70) |
| Bacteria + viruses + fungi + atypical pathogens (Mycoplasma or Chlamydia) | 9 (6.40) |
| Antibiotic exposure | |
| Yes | 87 (62.14) |
| No | 53 (37.86) |
The distribution of the different clinical confirmed pathogens in different seasons, different underlying diseases, different age groups and different types of LRTIs
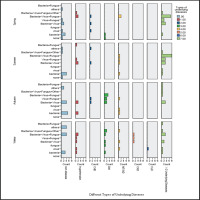
The proportion of LRTIs in the four seasons was basically consistent, about 33–39%, with no seasonal distribution differences. However, there were seasonal differences in the distribution of pathogens, with co-infections in spring and summer being the main part, and joint viral infections being the most common. In addition, the proportion of co-infections with more underlying diseases (≥2underlying diseases) was the highest, about 36.0%, followed by DM. Among them, the type of co-infection of bacteria, viruses, and fungi had the highest proportion, followed by bacterial combined with virus infection. The above results are detailed in Figure 2. Combining age, underlying diseases, and different types of LRTIs analysis, it was found that in advanced age (aged ≥80 years), multiple underlying diseases (≥2 underlying diseases), and severe LRTI named as AECOPD, the coinfection of bacteria, viruses, and fungi was the most significant, followed by CAP/P in 2 type DM. In addition, single fungal infections were rare, with only one case caused by a rare pathogen called Talaromyces, and only two such rare cases were included in the study (Figure 3).
The distribution of specific pathogenic microorganisms detected by t-NGS in 140 cases of BALF
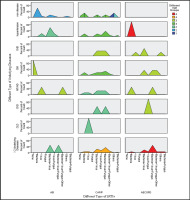
Overall, among the 140-case enrolled patients, the potential pathogens detectable by t-NGS in the BALF were bacteria, with the highest proportion 57.9% (81/140), followed by viruses 45.0% (63/140) and fungi 44.3% (62/140), and the lowest proportion was atypical pathogen including Mycoplasma and Chlamydia 10.7% (15/140). In addition, Mycobacterium tuberculosis complex as a type of bacteria, which was difficult to detect by CMTs in clinical practice, showed a significant positive detection rate of 11.4% (16/140). And Non-tuberculous mycobacteria (NTM) which refer to all Mycobacteria other than Mycobacterium tuberculosis and Mycobacterium leprae, also showed a higher detection rate, with 16 cases, accounting for 11.4% (16/140) and for 63.2% in advanced age patients (≥80). Among all pathogens, Haemophilus influenzae was the most common in bacteria, Epstein-Barr virus (EBV) was the most common in viruses, and Candida albicans was the most common in fungi. In addition, pathogens were classified as Class A, B and C. The above results are detailed in Supplementary file Figure 2.
The comparison and analysis of the results of detectable pathogen count by t-NGS and CMT
Overall, there were 140-case patients tested through t-NGS detection, among them positive results including 81 cases of bacteria, 63 cases of viruses, 62 cases of fungi; and 15 cases of other pathogens including Mycoplasma and Chlamydia. The total number of positive cases was 137 with a positive detection rate of 97.9% (137/140), and 105 cases were clinically consistent with FCCP (Supplementary file Figure 3). Through CMTs detection of pathogens, a total of 140 patients were tested, with 47 positive cases. Among them, there were 26 cases of bacteria, 12 cases of viruses, 1 case of fungi, and 8 cases of other pathogens. The total number of positive cases was 47 with a positive detection rate of 33.6% (47/140), and only one case was not clinically consistent with FCCP. The agreement test between the detection results of two different pathogen detection methods and FCCP showed that in the t-NGS detection method, the consistency between the detection results of various pathogens and FCCP was significant, with all kappa coefficients greater than 0.80. Among them, the t-NGS detection results of fungi had the highest consistency rate with FCCP, with a kappa coefficient of 0.92 and while other atypical pathogens, including Mycoplasma pneumoniae and Chlamydia psittaci, had the lowest. In addition, the consistency between various pathogens found in CMTs detection methods and FCCP is poor (all p>0.411), except for atypical pathogen (p=0.000). In addition, comparing the coincidence rate of different types of pathogens using different detection methods with FCCP, it was found that the t-NGS had the highest proportion of viruses (92.1%), bacterial (88.4%), and fungi (38.7%), while CMTs had the highest proportion of AP (80.0%), bacterial (34.2%), and fungi (4.2%). The overall consistency rate of t-NGS and CMTs were 77.1% and 43.8%, respectively. We also found that the accuracy of detecting pathogens by t-NGS method was significantly higher than that of CMTs method (all p≤0.001). However, overall the agreement test between t-NGS and CMTs finally showed a very low kappa coefficient (κ=0.31). The results are detailed in Table 2 and Supplementary file Figure 1.
Table 2
Comparison and analysis of detectable pathogen count by t-NGS and CMT
| Pathogen | Pathogen detection methods, n | FCCP N | Agreement test | Comparison of % accuracy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t-NGS | CMT | CMT | ||||||||||||
| BC | SMC | PCR | G-Test | CAD | T-Spot | TSCIR-T | κ | χ2/pa | χ2/pb | |||||
| Bacteria | 81 | 5 | 23 | - | - | - | 6 | 10 | 26 | 76 | 0.82* | 0.58** | 58.483/0.000 | 0.299/0.584 |
| Viruses | 63 | 0 | 0 | 6 | - | - | - | - | 12 | 58 | 0.89* | 0.25** | 128.430/0.000 | 0.060/0.807 |
| Fungi | 62 | 0 | 2 | 0 | 1 | 2 | - | - | 1 | 24 | 0.92* | 0.12** | 62.024/0.000 | 0.015/0.904 |
| AP (My/Ch) | 15 | 0 | 0 | 8 | - | - | - | - | 8 | 10 | 0.62* | 0.86** | 18.672/0.001 | 24.582/0.001 |
| PND | 137 | 5 | 23 | 14 | 1 | 2 | 6 | 10 | 47 | 105 | 0.31*** | 92.657/0.000 | 0.676/0.411 | |
| OSST | 140 | 33 | 123 | 48 | 24 | 25 | 40 | 31 | 140 | - | - | - | - | |
| PDR (%) | 97.86 | 15.15 | 18.70 | 77.78 | 4.17 | 8.00 | 15.00 | 32.26 | 33.57 | - | - | - | - | |
AP: atypical pathogens. FCCP: final clinical confirmed pathogen. My: mycoplasma. Ch: chlamydia. G-Test: serum 1, 3-beta-d-glucan level. n: the count number of positive. N: the count number of final clinic confirmed pathogen. CAD: cryptococcus antigen detection. BC: blood culture. SMC: sputum microscopy and culture. T-Spot: tuberculosis sport test. TSCIR-T: tuberculosis specific cellular immune response test.
*** Kappa coefficient based on agreement test between t-NGS test results and CMT. χ2/pa: Comparison of accuracy between t-NGS and FCCP by using Pearson chi-squared test. χ2/pb: Comparison of accuracy between CMT and FCCP by using Pearson chi-squared test. PND: positive number of detection. OSST: overall sample size for testing. PDR: positive detection rate.
Comparison of the antibiotic replacement rate of two different sample collection times of BALF
There was no significant difference in the replacement rate of antibiotics 3 days after admission between two different advanced ages and number of underlying diseases under the same sample collection time (SCT) (all p>0.15), while there was a difference observed among same age and the same underlying disease condition under the different SCTs (all p<0.01) (Table 3). This fully confirmed that although there are differences in the age distribution of pathogens, there was no statistically significant difference in the replacement rate of antibiotics beyond 3 days of admission under the same SCT, while there was a significant difference between the two different SCT. The same applies to underlying diseases.
Table 3
Comparison of the antibiotic replacement rate of two different sample collection times of BALF
[i] SCT: sample collection time. SUD: single underlying disease. pa: Comparison of antibiotic replacement rates under different ages and underlying diseases at the same sample collection time by Pearson chi-squared test. pb: Comparison of antibiotic replacement rates under same ages and underlying diseases at the different sample collection time by Pearson chi-squared test.
DISCUSSION
The clear diagnosis of the pathogens of LRTIs is essential for clinical treatment and reduction of serious complications and mortality of patients with LRTIs, especially for advanced age patients. Many previous studies indicated that the t-NGS technology employed here exhibits comparable performance to m-NGS in detecting respiratory pathogens while reducing costs by three-quarters8, highlighting the potential utility of t-NGS in pathogenic diagnosis13. In this study, among the enrolled 140-case patients with LRTIs, basic epidemiological characteristics showed that the proportions of age ≥60 years, male, CAP/pneumonia, underlying diseases (≥2), winter, and co-infection (bacteria + viruses + fungi) are the highest. In addition, a high level of antimicrobial exposure is 62.1%, this indirectly confirmed the epidemiological characteristics of LRTIs in advanced age adults at present14,15 including antibiotic misuse in respiratory tract infections caused by higher level of antimicrobial exposure16. By further analysis of the distribution of pathogens detected by t-NGS, we also found that in advanced age (≥80 years), multiple underlying diseases (≥2 underlying diseases), and severe LRTI named as AECOPD, the co-infection of viruses, bacteria, and fungi was the most significant, followed by CAP/pneumonia, especially for patients with 2 type DM combined with other underlying diseases17. In addition, there were also seasonal differences in the distribution of pathogens, with joint infections in spring and summer being major parts, and joint viral infections being the most common. However, a single fungal infection was extremely rare, and in this study we found only one case caused by Talaromyces marneffei infection, which occurred during the recovery period after upper respiratory tract infection. Another patient with Talaromyces marneffei infection had both type 2 DM and Graves’ disease as underlying diseases. At the same time, the clinical characteristics were fully consistent with their corresponding epidemiological features, such as a typical history of catching bamboo rats and a population with weakened immunity18. This was also fully consistent with the characteristics of Talaromyces marneffei infection reported in the literature19. The age distribution heterogeneity and that of underlying disease distribution can provide certain guidance value for empirical early use of antibiotics in subsequent clinical practice. As is well known, fungal infections in clinical practice are more common in patients with weakened immunity, long-term use of antibiotics16, or impaired immune function. Therefore, fungal infections were often a mixed infection of multiple pathogens, and this study also fully confirmed this phenomenon. Therefore, the determination of fungal infections in the lower respiratory tract is often based on clinical history characteristics related to immunological underlying diseases, especially for the immune-compromised patients, research showed that the antibiotic escalation in the immune-compromised group was more frequent than that in the immune-competent group16. Many studies have also confirmed that many fungi are particularly vulnerable to fungal infection in advanced age patients after infection with many different pathogens, such as pneumococcal infection20, HIV infection21, and COVID-19 infection22. However, the co-pathogenesis of respiratory viral and fungal co-infections is complex and involves a dynamic interplay between the host immune defenses and the virulence of the microbes involved that often results in failure to return to the homeostasis of pathogenic microorganisms15,23. This undoubtedly poses a huge challenge for clinical determination of fungal pathogen infections. Although the t-NGS detection method has increased the positivity rate of fungal pathogens, except for certain highly specific fungal pathogens, it is very difficult to determine and guide the clinical use of antibiotics.
Overall, t-NGS identified a greater number of potential pathogens cases (137 vs 47) compared with CMTs among 140-case enrolled patients, encompassing common or clinically relevant respiratory pathogens, including bacteria, viruses, fungi, and atypical pathogens. Moreover, t-NGS exhibited a higher positive detection rate compared to CMT (97.9% and 33.6%). Among them, as the gold standard for diagnosing pathogenic microorganisms, microbial culture, including blood and sputum culture, had a positive rate of only 15.2% and 18.7%, respectively. Such a low positive culture rate indicates that microbial culture can no longer meet the needs of clinical diagnosis, which is closely related to the high exposure rate of antibiotics before culture (62.1%), especially for patients with 2-type DM24. In addition, the limited cultivation techniques and conditions of grassroots hospital also greatly limited the positive rate of cultivation results.
By comparing the accuracy of detecting different pathogens, it was found that, except for atypical pathogens (Mycoplasma pneumoniae and Chlamydia psittaci), t-NGS was significantly higher than CMTs in detecting bacteria, viruses, and fungi, especially in detecting Mycobacterium tuberculosis and related drug resistance25,26, viruses, fungi and new colonization pathogens, all demonstrating the enormous superiority of t-NGS27,28. The consistency test of the two methods for detecting different pathogens also showed that compared with the CMT detection method, t-NGS showed good consistency, especially in fungi, followed by viruses. However, overall the agreement test between t-NGS and CMT finally showed a very low kappa coefficient. This indicated that although CMTs had a low positive detection rate as a routine method for pathogen detection in clinical practice, they could not be completely replaced by t-NGS. In addition, the comparison of the consistency of the two detection methods compared with FCCP also showed that t-NGS exhibited significant superiority in viruses and fungi. The overall consistency rates of the two detection methods were t-NGS and CMTs, respectively. This all indicated that t-NGS displayed great superiority in terms of pathogen detection28,29. There was research confirming that the overall consistency rates of t-NGS was 60.3%; however, the collected and tested samples were not BALF28, this may be the main reason for the decrease compared to this study. Another recent study also showed that the overall consistency rate of t-NGS was 64.2%30, this proportion was close to the results of this study (77.1%), as the majority of the samples tested were BALF. In this study, by indirectly analyzing the impact of different timing of t-NGS detection caused by different sample collection time on the clinical antibiotic replacement rate, we found that early use of t-NGS for pathogen detection can significantly reduce the antibiotic replacement rate, especially in elderly patients and patients with multiple underlying diseases. This result indirectly confirmed the guiding value of t-NGS in clinical practice, which fully conformed to the distribution characteristics of pathogen infections obtained in the previous section. In addition, early adoption of t-NGS for pathogen detection to guide clinical antibiotic use significantly reduces the rate of antibiotic replacement, which may indirectly shorten hospitalization time and lower hospitalization costs.
Although t-NGS has an unparalleled high positive detection rate, higher consistency and accuracy rate, it is sometimes very difficult to determine the pathogen of infection in clinical practice. The confirmed definition of most B-class pathogens is very difficult in clinical practice because the same class of pathogen may vary between class A and class B in different patients. In this study, we found that many B-class pathogens, such as Candida albicans, Cytomegalo virus (CMV) and Epstein-Barr virus (EBV), became potential pathogenic pathogens at different ages and with different underlying diseases, especially certain viruses (CMV), fungi (Candida albicans), and bacteria (Fusobacterium nucleatum). This was related to the weakened microbial regulatory immune response and colonization resistance in patients with advanced age (≥60 years) and multiple underlying diseases, and confirmed by related literature11,27,29. Although the bacterial DNA content in BALF is low, the composition of the lower respiratory tract microbiota often resembles that of the oropharyngeal microbiota, as the presence of a small number of surviving microorganisms in the lower respiratory tract of many healthy individuals is currently determined and confirmed31. However, it is currently unclear whether these microorganisms only represent a transient microbial community with sustained micro-aspiration and rapid elimination, or whether there are independent self-sustaining (local replication) microorganisms32. Therefore, establishing an effective t-NGS diagnostic evaluation process is a key measure to address the certainty of pathogen susceptibility in different categories (A, B, or C), especially for advanced age patients with multiple underlying diseases and immune-compromised populations because the antibiotic escalation in the immune-compromised group was more frequent than that in the immune-competent group33. In this study, we found that the confirmed diagnosis of pathogens between CCP and FCCP is not completely consistent, which fully demonstrates that it is very difficult to determine the diagnosis of certain pathogens in clinical practice. This requires many experienced senior physicians to combine imaging, dynamic detection of relevant inflammatory indicators, and clinical efficacy to comprehensively evaluate and ultimately determine. In addition, improving and further enhancing the detection and evaluation process of t-NGS may also be crucial. Anis et al.34 had all showed that the workflow of t-NGS applied to BALF specimens could enhance the detection rate of respiratory pathogens, although optimal approaches were not denied. Recently, the KingMed Diagnosis agency mentioned in the article discovered a new t-NGS method called hybrid capture-based targeted next-generation sequencing (hc-tNGS) for detecting pathogens in BALF, once again demonstrating the clinical feasibility of t-NGS35. With the continuous improvement and updating of this detection technology and workflow, it may bring great prospects for clinical detection and determination of infectious pathogens in the near future. Therefore, how to establish an efficient and reliable diagnostic workflow, especially how to evaluate and obtain clinical confirmation of the testing result of t-NGS, is crucial and currently the biggest challenge for clinical physicians.
In view of the above, the reliability and clinical practical value of t-NGS detection of pathogens by comparison and analysis of the distribution characteristics of pathogens detected by t-NGS and CMTs for LRTIs in different age groups, seasons, underlying diseases, and different disease types of LRTIs, and the consistency and accuracy of confirmed different pathogens detected by t-NGS and CMTs, our study underscores the clinical utility of BALF-based t-NGS in the pathogenic diagnosis of LRTIs among patients who are candidates for BALF. Especially in grassroots hospitals, it is particularly important for advanced patients and multiple underlying diseases with LRTIs. Early use of t-NGS for pathogen detection may provide better guidance for clinical antibiotic use.
Strengths and limitations
This study is currently the only one that uses t-NGS and CMTs test results from patients with the same lower respiratory tract infection for clinical comparison. The clinical matching is completely consistent, and the results have certain guiding significance. So far, the lack of reference standards for definitive diagnosis of pathogens in clinical practice has hindered the calculation of t-NGS sensitivity and specificity, thereby hindering the comprehensive evaluation of its diagnostic performance. In addition, although microbial cultivation is currently the gold standard for pathogen diagnosis, its low positivity rate is the biggest challenge, especially in primary hospitals. Furthermore, to effectively confirm the infectious pathogens one needs to study the combined results of t-NGS and the clinical practice based on CMTs and imaging characteristics and clinical therapeutic effect.
The purpose of this study was to investigate the clinical practicality of t-NGS. Although the study involved a high positive detection rate, consistency, and accuracy of t-NGS in clinical pathogen diagnosis, as well as the impact of antibiotic replacement rates, it did not extensively explore the clinical practical value of t-NGS, such as the economic cost-effectiveness of patient hospitalization time and expenses.
CONCLUSIONS
Due to its suitability for the distribution characteristics of clinical pathogens, high positive detection rate, clinical consistency, accuracy, and low antibiotic replacement rate in advanced age patients with LRTIs, t-NGS detection method has great clinical prospects for use in primary hospitals. However, how to confirm pathogens is the biggest obstacle and challenge in clinical practice.