INTRODUCTION
SARS-CoV-2 infection has continued to affect many patients since December 2019, when it was first detected. Although most children with SARS-CoV-2 infection present acutely with mild symptoms, vigilance is required for potentially serious complications. Fever, cough, and dyspnea are the most reported respiratory symptoms in children1. Spontaneous pneumothorax occurs without any obvious trauma or iatrogenic cause and is classified as primary or secondary. In primary spontaneous pneumothorax, there is an absence of known lung disease, whereas secondary presents as a complication of underlying pulmonary disease2. Spontaneous pneumothorax is an unusual but potentially life-threatening complication of SARS-CoV-2, with an overall incidence of 5–10 cases per 100000 children younger than 18 years3. Spontaneous pneumothorax without evidence of concurrent pneumonia or mechanical injury in children with SARS-CoV-2 infection is not often reported4. We describe the case of a 16-year-old boy with pneumothorax and no evidence of pneumonia two weeks after the acute phase of SARS-CoV-2 infection, and we review the relative literature.
CASE PRESENTATION
A 16-year-old boy, previously well, presented with chest pain on the right hemithorax lasting for two hours. He was afebrile, with a cough and respiratory distress. There was no history of trauma, vigorous exercise, or cough in the previous days. There was no history of asthma, cystic fibrosis and connective tissue disorder. Family history was clinically insignificant. Two weeks prior to admission, he reported low-grade fever and mild cough, and a rapid SARS-CoV-2 antigen test was positive. On clinical examination, he was tachypneic (respiration rate 40 breaths/min, SpO2 96%, 140 bpm), and on chest auscultation, absent lung sounds were detected on the right chest base.
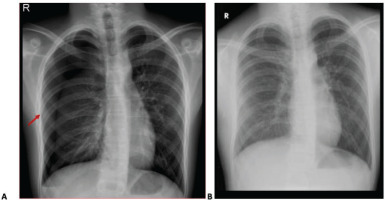
Lower respiratory tract infection was ruled out based on the absence of fever, raised inflammatory markers and consolidation on chest x-ray. Polymerase Chain Reaction (PCR) was positive for SARS-CoV-2, with a cycle threshold of ORF 26/N 27. Since there were no signs of respiratory distress and oxygen requirements, ARDS was excluded. Pulmonary embolism was a minor consideration, but the patient did not have any predisposing risk factors or CT chest findings consistent with it. Myocardial ischemia, myocarditis, pericarditis, and aortic dissection were included in the differential diagnosis. However, the pain characteristics, negative biochemistry (CK-MB, troponin), and normal ECG have excluded these diagnoses. Marfan syndrome was included in the differential diagnosis since the patient was tall and slender with a BMI of approximately 18 kg/m2. Nevertheless, a heart ECHO was performed and was reported as normal. Additionally, an ophthalmological examination excluded lens abnormalities. There was no pain on chest palpation; hence, the diagnosis of costochondritis was excluded. Chest X-ray has confirmed the presence of pneumothorax (Figure 1A). Based on the clinical history, examination and initial investigations, no further laboratory tests were performed for rheumatological and inflammatory conditions, since the diagnosis of secondary spontaneous pneumothorax associated with SARS-CoV-2 infection was made.
Subsequently, a chest drain was inserted, and symptoms improved. The chest drain remained for eight days till the complete resolution of pneumothorax (Figure 1 A and B).
DISCUSSION
Based on this case, we have conducted a literature review in PubMed and Google Scholar using the following search terms: SARS-CoV-2, COVID-19, spontaneous pneumothorax, pneumomediastinum, risk factors, predisposing factors, death, children, and adolescents. After searching for pneumothorax as a complication of SARS-CoV-2 infection, we have identified sixteen cases of children/adolescents with spontaneous pneumothorax and SARS-CoV-2 infection (Table 1)4-17.
Table 1
Reports of spontaneous pneumothorax and SARS-CoV-2 infection in children and adolescents
| Author Year | Age (years) | Sex | Coexisting conditions | Clinical presentation | CT findings | COVID-19 RT-PCR test | Time of presentation | Therapy | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Buonsenso et al.4 2021 | 17 | Male | Negative | Chest pain | Pneumothorax, pneumomediastinum, pneumorrachis | (+) | Incidental finding | Conservative | Yes |
| Musolino et al.5 2021 | 16 | Male | Negative | Chest pain, subcutaneous emphysema, ARDS | Pneumothorax, pneumomediastinum | (+) | Incidental finding | Conservative | Yes |
| Musolino et al.5 2021 | 17 | Male | Negative | Chest pain, respiratory distress | Pneumothorax | (+) | 10 days after asymptomatic COVID-19 infection | Pleural drainage with aspiration | Yes |
| LaaribiI et al.6 2022 | 9 months | Male | Epilepsy | Respiratory distress, fever, tachypnoea, O2 desaturation | Pneumothorax, alveolar pneumonitis consolidation | (+) | On admission | Pleural drainage | Yes |
| LaaribiI et al.6 2022 | 1.5 | Male | Negative | Dry cough, respiratory distress, fever, O2 desaturation | Alveolar – interstitial pneumonopathy, pneumothorax | (+) | On admission | Pleural drainage | Died due to urosepsis |
| Oterino et al.7 2020 | 6 | Female | Systemic sclerosis | Unknown | Pneumothorax, pneumomediastinum, interstitial emphysema and subcutaneous emphysema | (+) | Incidental finding | Conservative | Died due to respiratory failure |
| Carroll et al.8 2020 | 9 | Female | Medulloblastoma Neurosurgery | ARDS | Bilateral parahilar infiltrates and consolidation. Diffuse ground glass opacity in the right lung-Pneumomediastinum | (+) | Incidental finding | Conservative | Yes |
| Bellini et al.9 2020 | 17 | Male | Negative | Mild dyspnea | Pneumomediastinum | (+) | 2 weeks after acute infection | Nil | Yes |
| Karande et al.10 2021 | 8 | Male | Miliary Tuberculosis | ARDS | Bilateral pneumothorax | (-) | Unknown past infection | Intercostal drainage | Yes |
| Montgomery et al.11 2021 | 17 | Male | Negative | Chest pain, dyspnea | Hemo-pneumothorax | (+) | Incidental finding | Bleb dissection, intercostal drainage | Yes |
| Quintana-Ortega et al.12 2021 | 11 | Female | anti-MDA5 juvenile dermatomyositis | Dry cough, skin rash | Pneumothorax, pneumomediastinum | (+) | Onset of symptoms: 5 days before respiratory deterioration | Multiple immunosuppressive and antibiotic therapy | Died due to Multi-organ system failure |
| Hashemi et al.13 2021 | 2 | Male | Hyper IgM syndrome | Fever, cough, shortness of breath, tachypnoea | Pneumothorax | (+) | 4 days after symptomatology beginning | Conservative | Yes |
| Giné et al.14 2020 | 14 | Female | Allergy and asthma during infancy | Chest pain, fever, cough, anosmia, ageusia | Pneumothorax, bullae existence | (+) | 11 days after symptomatology beginning | Intercostal drainage, bullae dissection | Yes |
| Soyak et al.15 2022 | 15 | Male | Obesity, asthma | Cardiac arrest | Pneumothorax | Unknown | Unknown | Pleural drainage, mechanical ventilator support | Yes |
| Blondeau et al.16 2022 | 5 | Male | Negative | Cough, right shoulder pain | Pneumothorax, pneumonia prior of pneumothorax, multilobulated cystic pulmonary mass | (+) | 5 days after positive COVID test and beginning of symptomatology | Pleural drainage, antibiotic, antiviral therapy | Yes |
| Stewart et al.17 2022 | 13 | Male | Autism | Chest pain, cough | Pneumothorax, pneumonia prior to pneumothorax, multiple bullae formation | Unknown | 23 days after symptomatology beginning and on readmission | Pleural drainage | Yes |
Epidemiological and clinical characteristics of children with spontaneous pneumothorax following SARS-CoV-2 infection are presented in Table 1. Most of the reported children in the literature were males (12/16) with a mean age 10.6 ± 6 years and a median age of 12.0 years. Only four cases exhibited spontaneous pneumothorax after the onset of SARS-CoV-2 infection symptoms. The time interval between the beginning of infection symptoms and the pneumothorax presentation was 10 to 23 days. All these four patients were adolescents, but only one was female, who was also the only one with pre-existing conditions, such as allergies and asthma. Underlying medical conditions were described in 9/16 children.
Seven children were identified with symptoms of spontaneous pneumothorax without clearly specifying the chronological relationship between active infection and pneumothorax symptoms. Six of them had positive PCR for SARS-CoV-2, and the seventh had a negative test. The latter patient’s current clinical presentation was attributed to COVID-19 because of the existence of positive SARS-CoV-2 IgG serum antibodies. Seven of the patients were referred with chest pain, and ten with respiratory distress. Only two male patients presented with unilateral pneumothorax. A 17-year-old male had hemopneumothorax. In the other five reported cases, pneumothorax was combined with pneumomediastinum, and a 17-year-old male was diagnosed with pneumothorax, pneumomediastinum, and pneumorrachis. A 9-year-old female with a clinical history of medulloblastoma and a 9-month-old male with a history of epilepsy had consolidations and infiltrates along with pneumomediastinum. A 17-year-old adolescent had shown radiological signs of a lung infection after the onset of pneumothorax, and two patients had radiological findings of pneumonia prior to the appearance of pneumothorax. Three patients were reported with bullae coexistence. One patient had a history of miliary tuberculosis. One patient who had pre-existing asthma and obesity developed severe pneumothorax on the base of decreased lung function and was intubated and ventilated due to cardiorespiratory arrest. Three patients who died were females with associated connective tissue disorders, such as multiple sclerosis and juvenile dermatomyositis, and one was a male with urosepsis. Nine patients underwent intercostal drainage, and the rest of the reported cases (seven patients) were treated conservatively.
Here we reported the case of spontaneous pneumothorax following SARS-CoV-2 infection (secondary spontaneous pneumothorax). In the literature, several adult cases of delayed or secondary spontaneous pneumothorax following SARS-CoV-2 infection have been reported and are associated with SARS-CoV-2 pneumonia and mechanical ventilation4. Spontaneous pneumothorax has been documented only in a few cases of a previously healthy adolescent.
Most patients who developed pneumothorax associated with COVID-19 infection were males. In children, the incidence of spontaneous pneumothorax is estimated to be 4.0/100000 population per year in males and 1.1/100000 population per year in females18. Male predominance in spontaneous pneumothorax has been previously referred to in the literature with a female/male ratio of 1:3.319. Secondary pneumothorax has an even greater predominance in males.
In the published cases, nine patients were found to suffer from underlying conditions, but only in six of them, the underlying condition could have affected the lungs, hence predisposing them to pneumothorax. Two patients had asthma, and one patient had dermatomyositis, systemic sclerosis, hyper IgM syndrome, and miliary tuberculosis. Connective tissue disorders such as Marfan syndrome and Ehlers-Danlos syndrome, homocystinuria, asthma, cystic fibrosis, a1-antitrypsin deficiency, chronic obstructive pulmonary disease, cystic disorders of the lungs and malignancies are associated with pneumothorax20-23. Hyper IgM syndrome is associated with bronchiectasis and a decline in lung function, whereas scleroderma and dermatomyositis can be complicated by interstitial lung disease24,25. Medulloblastoma is associated with lung metastasis; nevertheless, this has not been reported in the case by Carroll et al.8. In adults, the incidence of spontaneous pneumothorax in all hospitalized patients is 0.3% to 1%, in hospitalized patients with pneumonia, it is 3%, and in patients who underwent mechanical ventilation, it is 12% to 23%26,27. However, the presence of SARS-CoV-2 infection in a patient with underlying pulmonary disease could have triggered pneumothorax. The pathophysiological mechanism is not clear, but the hyperproduction of cytokines and exaggerated immune response caused by the viral infection may contribute to pneumothorax forming, especially when there is underlying lung pathology26-28.
In the previously reported cases, two female patients with connective tissue disorders and pneumothorax died, whereas one male patient with immunodeficiency survived. Autoimmune diseases are known risk factors for SARS-CoV-2 complications due to the presence of a hyper-inflammatory state29. The two patients who suffered from juvenile dermatomyositis and systemic sclerosis had both severe predisposing lung involvement since the first had Pneumonocystis jirovecci infection and the second had respiratory complications of autoimmunity. Shields et al.19 have reported that adult patients with primary immunodeficiency and symptomatic secondary immunodeficiency display greater morbidity from SARS-CoV-2, particularly if they have associated chronic lung and/or liver disease. Nevertheless, the authors have reported that children with controlled immunodeficiency have lower morbidity compared to the adult population.
In the literature, there are no data to estimate the risk of death due to the coexistence of pneumothorax and SARS-CoV-2 infection in children. Data from adults report older age, male gender, obesity and comorbidities as risk factors for disease severity21,30. Laaribi et al.6, however, reported two cases of SARS-CoV-2 infection in infants and pneumothorax, but only one has survived. The infant who did not survive died from urosepsis, which can be explained by the susceptibility of the immature immune system to more severe inflammation and infection. In the study by Soyak et al.15, the presence of obesity and asthma in a 15-year-old male adolescent resulted in decreased lung function, which in turn was a risk factor for life-threatening pneumothorax in a child with SARS-Cov-2 infection. In many cases, a SARS-CoV-2 positive test is coincidental and is not considered a risk factor for severe disease due to pneumothorax30. The coexistence of neuro disability and respiratory condition further increases the mortality risk; nevertheless, the authors have not described in detail if pneumothorax was included in the respiratory condition31.
ARDS, dyspnea, and chest pain were reported as the most common presenting symptoms in adolescents. Adult patients report chest pain as the most specific and common symptom at the onset of the disease32.
CONCLUSION
Spontaneous pneumothorax is a very rare complication during SARS-CoV-2 infection in the pediatric population. It can present up to 4 weeks after the acute onset of the infection. In most cases, no history of preceding lung disease exists. Younger age, underlying respiratory conditions and connective tissue disorder are previously described as risk factors for more severe clinical presentation. Clinicians should be vigilant for pneumothorax diagnosis when a child presents with chest pain and positive SARS-CoV-2 infection history.