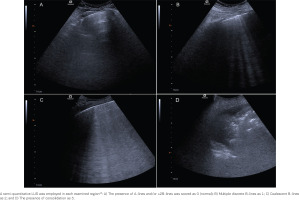
INTRODUCTION
Lung ultrasound has gained increasing importance as an imaging tool in the field of respiratory failure, due to its noninvasiveness, safety, and bedside availability1. Lung ultrasound enables ARDS diagnosis, with accuracy comparable to chest X-ray interpretation based on guidelines2, and the new proposed global definition of ARDS includes the use of ultrasound for the diagnosis of bilateral B-lines and/or consolidation3. Moreover, lung ultrasound outperforms chest X-ray for the differential diagnosis of pulmonary abnormalities in the ICU4 and can impact clinical decision making and therapeutic management5. Due to these advantages, lung ultrasound has especially been used during the COVID-19 pandemic6, allowing the detection of early disease7 and follow-up of patients8, while minimizing the need of transferring patients to the radiology department and, hence, reducing virus transmission. Moreover, in COVID-19 pneumonia, a lung ultrasound score (LUS) was associated with the disease severity, as assessed by chest CT and clinical features9, the estimation of extravascular lung water10 and the aeration following prone position11, while it has been shown to enable monitoring of PEEP-induced recruitment in mechanically ventilated patients with ARDS12.
As a relatively new technology, lung ultrasound has possible pitfalls13,14 and a limitation of ultrasound is that it is a technique with high operator dependence15. This is an important factor for the implementation of ultrasound in ‘real-life’ clinical practice, especially in the critically ill patient, where clinical instability may require serial monitoring from different attending physicians. Indeed, in previous large observational studies, lung ultrasound was performed by specified trained operators16.
Thus, the aim of our study was to assess the utility of lung ultrasound performed by different physicians in a multi-center observational study and to correlate lung ultrasound with important outcomes in critically ill patients with COVID-19 pneumonia.
METHODS
Subjects
Intubated patients due to COVID-19 pneumonia were included in the study. All patients had a positive SARS-COV-2 polymerase chain reaction (PCR) test in bronchial secretions and/or in nasopharyngeal swab. Data were acquired from the multi-center Greek RECUP-19 (Registry of Hellenic ICUs for Pandemic COVID-19) database that prospectively follows patients with COVID-19 pneumonia hospitalized in ICU. The ICU departments of ‘Sotiria’ Chest Hospital, University Hospital of Heraklion and ‘George Papanikolaou’ General Hospital of Thessaloniki participated in the study. The study protocol was approved by the Scientific Committee of the participating hospitals. Due to the observational nature of the study, which only collected clinical data from the patients’ record, informed consent was waived.
Lung ultrasound protocol
Lung ultrasound was performed as part of the clinical evaluation, within 24 hours upon admission from the attending physician. No examination was performed during or after a prone positioning session. Lung ultrasound was performed on the patient at the supine posture with 30° inclination, using the phased array or the convex (abdominal) probe, according to Mojoli et al.17. Each hemithorax was divided into three areas, anterior, lateral, and posterior, defined by the sternum, the anterior, and posterior maxillary lines, respectively. Each region was further divided into an upper and lower zone, creating 6 regions of interest by hemithorax. Lung ultrasound clips were stored for further quantification. Lung ultrasound patterns during a complete respiratory cycle were identified and scored using a semi-quantitative scale, based on the worst identified pattern12, as follows: 0 = Α lines and/or ≤ 2B-lines (normal), 1 = multiple discrete B lines (moderate loss of aeration), 2 = multiple coalescent B lines (severe loss of aeration), 3 = consolidation (complete loss of aeration). The scores from each region were summed creating a final lung ultrasound score (LUS) ranging from 0 to 3612,16,17. To avoid overestimation, a score of 3 was only given when consolidation was the dominant ultrasound image17.
Statistical analysis
Data are presented as mean ± standard deviation. Normality of our data was assessed using the Kolmogorov-Smirnov test. Comparisons between two groups were performed with Student’s t-test, and correlations between variables were assessed with the Pearson r correlation coefficient. A p<0.05 was used to denote statistical significance.
RESULTS
Subjects
Our study included 29 patients with severe COVID-19 pneumonia under mechanical ventilation, with a mean age of 67.07 ± 13.69 years. The APACHE-II mean score upon admission was 15.62 ± 6.08. Baseline characteristics, including parameters of the mechanical ventilation settings, are presented in Table 1. Mean LUS was 19.83 ± 6.01. From 348 regions scanned (29 patients × 12 regions), 8.05% (n=28) were scored as 0, 26.15% (n=91) were scored as 1, 51.72% (n=180) were scored as 2, and 8.05% (n=28) were scored as 3. Thus, the presence of multiple B-lines (either discrete or coalescent) was the most frequent finding in our cohort, with 12 patients (41.38%) having at least 1 region scored as 3, i.e. consolidation pattern.
Table 1
Physiological characteristics of patients upon admission
The relationship between LUS and mortality
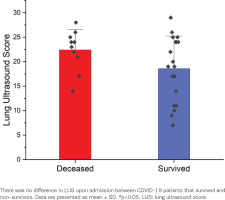
Our cohort presented a 28-day mortality rate of 34.5%. The lung ultrasound score (LUS) upon admission did not differ significantly between survivors and non-survivors (18.58 ± 6.62 vs 22.4 ± 4.20, p=0.11) (Figure 2).
The correlation of LUS with physiological parameters upon admission
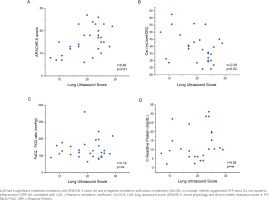
As shown in Figure 3, the LUS upon ICU admission has a significant moderate correlation with APACHE-II score (r=0.49, p=0.006) and a significant negative moderate correlation with the static compliance of the respiratory system (r= -0.39, p=0.03). In contrast, no correlation was found with systemic inflammation, as estimated by the c-reactive protein (CRP) (r=0.19, p=0.35) or with the oxygenation, as estimated by the P:F ratio (r=0.05, p=0.78).
Estimation of spatial differences in LUS and relationship with respiratory system static compliance
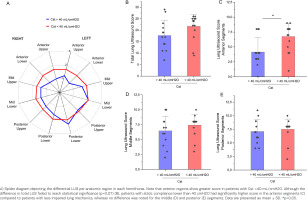
A separate analysis was performed using the cut-off value of 40 mL/cmH2O for static lung compliance, as this has been previously suggested to define severe alteration in lung mechanics18. As shown in Figure 4, patients with preserved/mildly deranged static lung compliance (Cst ≥40 mL/cmH2O) had a differential lung ultrasound score per anatomical region, compared to patients with Cst <40 mL/cmH2O. In details, mildly deranged lung mechanics were associated with reduced LUS in the anterior segments of the lung, compared to LUS in anterior segments of patients with reduced Cst (4.15 ± 2.44 vs 6.75 ± 2.20, p=0.006), whereas no difference was observed for the posterior segments (p=0.58).
DISCUSSION
The main findings of our study are that lung ultrasound upon admission does not predict survival in critically ill patients with COVID-19 pneumonia. However, significant correlations with the APACHE-II score and the mechanical properties of the respiratory system are noted. Moreover, reduced static compliance is associated with higher lung ultrasound score in the anterior segments, compared to preserved static compliance.
The lack of a difference of LUS between survivors versus non-survivors in our study contrasts with a previous study, where lung ultrasound upon hospital admission was a strong predictor of mortality in COVID-19 patients16. However, this study included patients across all disease stages, whereas in our study we included only critically ill patients in the ICU. Mortality in critically ill COVID-19 patients is shown to be multifactorial, with multiorgan dysfunction syndrome related to both COVID-19 or secondary infections being the most common cause of death in the ICU19, whereas a minority of patients die from refractory hypoxemia. We also acknowledge that a limitation of our study is the small number of patients that may reduce the power of the study to detect a significant correlation to mortality. Indeed, given the moderate correlation of LUS with the APACHE-II score, it is possible that a significant difference with mortality would be detected, given that a previous retrospective study found that the APACHE-II score upon admission was significantly different between survivors versus non-survivors in COVID-19 pneumonia in the ICU20. Therefore, survival of COVID-19 critically ill patients is dependent on many factors and admission LUS could potentially be among them but not the only one.
An interesting finding of our study is the correlation of lung mechanics with LUS in critically ill patients. This may be of additional value in groups of patients, in whom assessment of the mechanical properties is challenging, such as patients on non-invasive mechanical ventilation or patients on partial support ventilation. Identifying low compliance is, however, essential, since it allows the monitoring of critically ill patients and may unmask high respiratory drive21 and increased work of breathing, which may necessitate invasive mechanical ventilation or lead to lung and diaphragm injurious ventilatory settings22. Furthermore, the positive correlation between respiratory system mechanics and LUS can be useful in predicting the responders to recruitment maneuvers. According to the most recently published ESICM guidelines for acute respiratory distress syndrome (ARDS)23, there is a strong recommendation against the routine use of high-pressure recruitment maneuvers in ARDS patients and lung ultrasound could be valuable in recognizing the patients that will benefit for these strategies and enable a ‘precision medicine’ approach.
Despite the correlation of lung ultrasound with lung mechanics, it is of interest to notice the lack of any correlation with oxygenation. Although the reason for this cannot be addressed by our study, some assumptions can be made. Indeed, the contribution of vascular abnormalities to ventilation/perfusion mismatches, which have been found in COVID-19 patients24, would not be identified by lung ultrasound. Moreover, the presence of overdistension and dead space would not contribute to lung ultrasound score12, although it significantly impacts gas exchange and oxygenation in mechanically ventilated patients25. Our observation that LUS is correlated with the compliance of the respiratory system and not with oxygenation, is in accordance with the mentioned dissociation between compliance and oxygenation in ARDS patients due to COVID-1926. Furthermore, the same dissociation was observed retrospectively in ARDS before the COVID-19 pandemic27 and not only in the early phase of ARDS, but also in persistent ARDS28. The lack of LUS correlation with CRP, a systemic marker of inflammation, contrasts with previous studies where LUS was positively associated with CRP levels29,30. However, these studies were performed in patients admitted to the hospital ward, whereas in our study critically ill patients were included upon ICU admission, therefore CRP levels could be affected by prior in-hospital treatments.
Our results also raise the intriguing possibility that lung ultrasound may be used to assess focal distribution of lung injury with interactions with lung mechanics. As shown, severe derangement of static lung compliance was associated with increased LUS in the anterior segments of the lung. Thus, a spatial analysis of lung ultrasound, together with the overall score, may further assist in identifying patients with severely impaired lung physiology. This is a further advantage of ultrasound, since focal distribution is currently assessed by CT imaging, that is associated with radiation exposure and a need for transportation or by technologies such as the electrical impedance tomography31,32, which, although helpful, is not widely available in clinical practice. Previously, spatial analysis of lung ultrasound and a reduction of lung ultrasound score in dorsal regions after prone position was able to identify patients with a positive response to prone position11.
CONCLUSIONS
Lung ultrasound performed under ‘real-life’ conditions adds valuable information in the clinical evaluation of critically ill patients with COVID-19 pneumonia in the ICU, although it is not associated with overall 28-day mortality. Our data support the implementation of lung ultrasound in clinical practice within the ICU, even (or especially) under crises, like the recent COVID-19 pandemic. Strategies to increase the awareness of the utility of lung ultrasound and widen its current use, through structured educational activities should be undertaken.