INTRODUCTION
The SARS-CoV-2 pandemic, known as COVID-19, has affected more than 94 million people globally, to date. The risk of thrombosis is increased in these patients. In a United States registry of patients with COVID-19, thrombotic complications occurred in 35.3% of hospitalized critically-ill patients1. However, spontaneous pneumomediastinum (PNM) and subcutaneous emphysema (SE) are rare complications with limited reported cases in the international literature to date2.
CASE PRESENTATION
A man aged 56 years, ex-smoker with an unremarkable medical background, presented to the Emergency Room with a six-day history of fever and dyspnea. The real-time reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2 was positive three days before. On physical examination, he had a low-grade temperature of 37.6℃, tachypnea (RR=35/min) and desaturation (SpO2 88% on FiO2 0.21). The rest of his vital signs were as follows: blood pressure of 130/70 mmHg and heart rate 85 beats per minute. During auscultation, he had crackles at the base of the lungs bilaterally. Chest x-ray showed widespread pulmonary infiltrates (Figure 1a). The baseline laboratory blood tests showed raised inflammatory markers suggestive of acute infection (Table 1). On admission, we used the standard therapy with intravenous azithromycin, and dexamethasone 8 mg/d, subcutaneous prophylactic low molecular weight heparin (LMWH), and supplemental oxygen via nasal cannula (6 L/min).
Table 1
Laboratory markers on admission and throughout hospitalization
Figure 1
a) PA chest x-ray on admission with COVID-19 widespread pulmonary infiltrates bilaterally. b) Subcutaneous emphysema (vertical lucent stripes into the neck) and “double bronchial wall sign” suggesting pneumomediastinum. Small line of air parallel to trachea and the left cardiac border
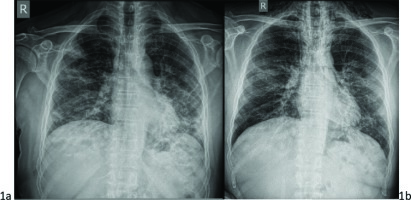
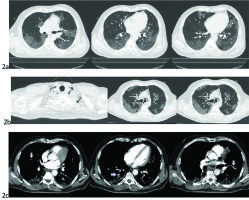
A chest computed tomography (CT) was performed that showed diffuse ground-glass infiltrations in the basal part of the lower lobes (Figure 2a). On the third hospitalization day, intravenous remdesivir was added to the therapeutic scheme following a decrease in the aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (98 IU/L and 112 IU/L, respectively). His condition deteriorated 3 days later with fever up to 38.5℃, gradual increase in oxygen demands, and raised inflammatory markers. A new chest x-ray was ordered on the sixth hospitalization day, which revealed cervical subcutaneous emphysema and radiolucent shades parallel to trachea and the left cardiac border. The latter is a characteristic feature of pneumomediastinum, known as ‘double wall sign’ (Figure 1b). These findings were confirmed by a new chest CT scan (Figure 2b). In addition, it showed interstitial emphysema with a small amount of air around the bronchi and pulmonary vessels in the area of the left hilum due to bronchial or alveolar rupture.
Figure 2
a) CT on day 3 admission with typical ground-glass pulmonary infiltrations suggestive of COVID pneumonia primarily affecting the basal parts of both lungs. b) CT showing widespread subcutaneous cervical emphysema around the strap muscles, and around the trachea in the superior mediastinum. Signs are suggestive of pneumomediastinum. c) CTPA showing pulmonary embolism in peripheral segmental branches of the pulmonary artery at different levels of cross sections (silver arrows)
We escalated the oxygen delivery method to a Venturi mask of FiO2 0.4, and initiated empirical double intravenous antibiotic therapy with piperacillin/tazobactam and vancomycin to prevent presumed mediastinitis. This antimicrobial treatment was based on the fact that he was already on the sixth day of hospitalization, as well as on the most up-to-date data for COVID-19 coinfections3. Therefore, Pseudomonas aeruginosa 4 and Methicillin-resistant Staphylococcus aureus (MRSA) 5 had to be covered, while the results from the blood cultures were still pending.
Of note, the patient had no risk factors for development of barotrauma as he did not undergo any procedures (e.g. central venous catheter insertion, or high-flow invasive or non-invasive mechanical ventilation), which would justify his decline. Also, there were no findings of emphysema in the lung parenchyma, such as subpleural bullae, which would theoretically rupture. In the following monitoring period, the SE was marked to the neck and the assessment of its progression was made by the presence of crepitus on palpation.
On the eleventh day of hospitalization, the patient complained of shortness of breath associated with pain in the left calf. A significant increase (×10) in d-dimers value of 36000 was noted (normal range <500 μg/L). The patient’s oxygen demands were elevated, the ECG revealed sinus rhythm and tachycardia while the SE had almost completely resolved clinically. A computed tomography pulmonary angiography (CTPA) confirmed the diagnosis of pulmonary embolism (PE); filling defects were observed in the segmental and subsegmental branches of the pulmonary artery (PA) supplying the middle and lower lobes bilaterally (Figure 2c). We emphasize that the SE and the presence of free air in the anterior mediastinum had resolved within 5 days. However, the ground-glass infiltrations worsened with localized consolidations in the lungs. The patient was immediately commenced on a therapeutic dose of LMWH (tinzaparine 175 iu/kg OD: 175×80 kg =14000 iu/d).
Within the following days, there was a remarkable overall clinical improvement as his oxygen demands became minimal, there were no spikes of fever and he remained hemodynamically stable during the rest of his hospitalization. He was discharged home with instructions to continue a course of therapeutic LMWH. An outpatient follow-up appointment was scheduled to evaluate his clinical progress in a month.
DISCUSSION
Pneumomediastinum is a rare complication of pneumonia due to COVID-19. There are two categories: primary (or spontaneous), and secondary (or traumatic), which may result from medical interventions6,7. Men are more often affected than women, with dyspnea and pleuritic chest pain being the most common presenting symptoms. Clinical signs include tachycardia, Hamman’s sign, ECG changes (i.e. premature contractions, inverted T-waves, low voltage readings), and rarely tension physiology (tension PNM) from direct cardiac and lung compression7. Chest radiograph and CT are the appropriate diagnostic tools for pneumomediastinum. If missed, more severe complications like pneumopericardium may result causing tamponade and mediastinitis7. Early diagnosis is essential for a proper treatment as the mortality is high.
Factors predisposing to air leak include alveolar rupture secondary to trauma or differences in pressure gradient, such as in cough, vomiting and other Valsava maneuvers, as well as inflammatory processes of the alveolar wall itself. Many researchers advocate that the pathophysiology of pneumomediastinum in COVID-19 patients is related to diffuse alveolar damage (DAD), cellular fibromyositis exudates, evident desquamation of pneumocytes and hyaline membrane formation2,6. As a result, gas leaks into the pulmonary interstitium, therefore causing interstitial emphysema6. The same findings were noticed in the CT scan of our patient. Pneumomediastinum may also be associated with pneumopericardium, and/or pneumothorax (PTX)2,6.
It is postulated that the virus disrupts the vascular endothelium by direct viral penetration into the endothelial cells in a similar manner as it does in the alveoli. Thus, it is believed that the PE as a complication in COVID-19 patients results from endothelial injury, which in turn releases cytokines and von Willebrand factor (VWF)8. These proinflammatory cytokines promote vascular endothelial cell apoptosis, pulmonary microthrombosis, vascular leakage and alveolar edema9. In addition, cytokine storm, macrophages, thrombin, platelets, monocytes, lymphocytes, and VWF, contribute to a hypercoagulable state in COVID-19. Lastly, hypoxia predisposes to thrombosis due to blood hyperviscosity10.
Despite the administration of prophylactic antithrombotic treatment in COVID-19 patients, the incidence of pulmonary embolism (PE) has been reported twice as high, especially in the patients admitted in the intensive care unit for supportive care due to COVID-19 pneumonia11. A systematic review by Liao et al.12 reported that nearly 2 in 10 patients developed PE from a total of 1835 COVID-19 patients. Preliminary studies in COVID-19 cohorts support that the prevalence of PE in these patients may range from 23 to 30%. The same studies showed that contrast-enhanced computed tomography pulmonary angiography (CTPA) is the gold standard for the diagnosis of PE13,14.
To the best of our knowledge, there are no published COVID-19 cases complicated by PE with concomitant pneumomediastinum. Perhaps, undetectable microemboli in peripheral branches of the PA occurred14. However, there are published COVID-19 cases with merely PNM (n=6; 66% males), and other cases with a combination of PNM and PTX (n=5)15. Radiological findings of subcutaneous emphysema were present in three out of the six patients with PNM, and in two out of the five patients with PNM/PTX4. Similarly, to our case, no specific treatment was required since the symptoms were self-limited within a short period of time. The outcome was favorable in 50% of the patients. However, 22.2% of the patients who passed away suffered from severe comorbidities4.
It is speculated that the prognosis in patients with PNM depends on the extent of the pulmonary injury2. We noticed in this particular case that the development of the pneumomediastinum coincided with the deterioration of pre-existing ground-glass infiltrations and localized ‘crazy paving’ pattern in the lower lobes, which did not pre-exist. Also, an elevated lactate dehydrogenase (LDH) is possibly a risk factor with prognostic value for patients with PNM2,6. Similarly, an elevated LDH (mean 863 U/L) was associated with poorer prognosis in SARS patients. In our case, the development of pneumomediastinum coincided with a doubling of the LDH value from 500 to 1000 U/L, and subsequent gradual decrease. The appearance of PNM was clinically subtle on the 12th day from the onset of symptoms, and compared to other cases, it was rather of early onset as usually it may occur late, between the 18th and 27th day of symptoms6.
CONCLUSION
Clinicians should suspect both PE and PNM within the differential diagnosis in cases of COVID-19 patients with pleuritic pain, dyspnea, and respiratory failure, after the tenth day from the onset of the symptoms. The simultaneous presence of these conditions in a patient may be life-threatening in an already compromised patient due to COVID-19 pneumonia. Clinicians must become aware of this novel presentation and initiate the proper treatment immediately.


