INTRODUCTION
Epidemiology, risk factors related to the patient and/or to the procedure/type of anaesthesia
Preoperative evaluation is a mandatory procedure that precedes surgical anaesthesia. It focuses on the respiratory and cardiovascular system, while on a per patient basis it takes into account specific comorbidities, e.g. malignancy, chronic diseases, and renal failure. It aims to assess the risk associated with each surgical intervention and to thus aid the selection of the appropriate plan of anaesthesia and of perioperative care for optimal patient safety. According to estimates, 14% of the complications related to anaesthesia and to surgical procedures, and 28% of the deaths attributed to anaesthesia, have been associated with an inadequate preoperative evaluation. Of note, comorbidities are frequent in patients undergoing preoperative evaluation (71%)1.
Risk factors related to the patient and the type of intervention are more important for the occurrence of complications than factors related to anaesthesia. Nonetheless, the selection of general or local anaesthesia constitutes a decision of major importance.
Risk factors for the occurrence of perioperative and postoperative complications include age >65 years, the presence of comorbidities, male gender, emergency surgery and the admission of patients during the weekend. The risk is clearly higher for cardiothoracic surgical procedures2,3.
A broad definition of postoperative complication includes an identifiable disease or dysfunction, resulting from the surgical procedure and the given anaesthesia that is clinically significant and affects patient outcome3,4. Postoperative pulmonary complications contribute significantly to perioperative morbidity and mortality (incidence: 3.1–9%). This definition includes entities such as atelectasis, infection (bronchitis and pneumonia), prolonged mechanical ventilation, respiratory failure, exacerbation of a pre-existing chronic respiratory disease (chronic obstructive pulmonary disease [COPD], asthma, bronchiectasis) and bronchospasm4. Risk factors for postoperative pulmonary complications can be classified as patient-related and procedure-related2,3,5.
Patient-related risk factors
Chronic respiratory disease constitutes the most important risk factor, increasing the risk of postoperative pulmonary complications by approximately 3-fold4.
Smoking is a risk factor for postoperative complications regardless of whether the patients have chronic respiratory disease. Smokers with >20 pack-years have 1.5–5.5% higher incidence of complications. Moreover, the relative risk of pulmonary complications in active smokers is four times higher compared to former smokers4,6.
Increased body weight is associated with an increased risk for postoperative complications due to the functional respiratory disorders and other comorbidities with which it is associated4-6.
Procedure-related risk factors
The site of the surgery constitutes the most important predictive factor for the cumulative risk of postoperative pulmonary complications. The likelihood of the complications is inversely proportional to the distance of the surgical incision from the septum. Therefore, the incidence of complications is higher for surgical procedures of the chest (19–59%) and the upper abdomen (17–19%), compared to surgical procedures involving the lower abdomen (0–5%)2,4.
The duration of the surgery is an important risk factor, with procedures of 3–4 h associated with a higher risk of pulmonary complications2,5,6.
The type of anaesthesia: epidural anaesthesia is associated with decreased mortality, reduced risk for myocardial infarction, lower likelihood of requiring blood transfusion, lower incidence of pneumonia and respiratory depression, reduced risk of inducing deep vein thrombosis and, in general, a thrombophilic condition when compared to general anaesthesia2,4-6.
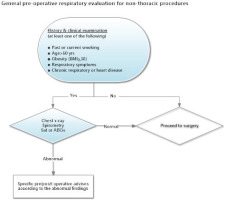
The majority of the patients undergo numerous preoperative investigations, in order to assess pre-existing health issues, to identify those that have not been diagnosed and to assess preoperative and postoperative complications. In general, targeted preoperative screening, based on the medical history and clinical examination, is preferable. Unnecessary investigations, accounting for 60–70% of all investigations, which cause delays in surgical interventions, high costs, and patient discomfort, should be avoided. Therefore, proper selection, through clearly delineated instructions, of the patients as well as of the range and type of the investigations that the patients will undergo preoperatively to assess the respiratory system are deemed necessary. A general algorithm for preoperative evaluation for surgical procedures apart from thoracotomy is illustrated in Figure 1.
GUIDELINES FOR THE PREOPERATIVE EVALUATION OF THE RESPIRATORY SYSTEM
General evaluation
History and physical examination
Obtaining a detailed medical history and performance of a comprehensive physical examination are essential in all patients to evaluate the pulmonary reserve. It is important to identify signs and symptoms of respiratory and non-respiratory diseases (such as heart failure [HF] and renal failure) or other systemic diseases related to the respiratory system, e.g. connective tissue diseases, etc. Medical history taking should include quantification of smoking habits, intake of medications with known toxicity to the lungs (nitrofurantoin, amiodarone, bleomycin), as well as history of environmental/occupational exposure (passive smoking, coal, asbestos).
Symptomatology indicative of sleep apnoea syndrome (chronic somnolence, snoring, observed episodes of apnoea), restriction of activities or the ability to exercise, pre-existing respiratory disease, recent respiratory infection and/or exacerbation of respiratory disease should be recorded in detail. During the patients’ preoperative evaluation, the detection and treatment of obstructive sleep apnoea (OSA) is of utmost importance, since it is associated with an increased risk of perioperative and postoperative complications. Although the method of choice for the identification of OSA is polysomnography, specific questionnaires have been developed as screening tools. Classification of physical activity as per the American Society of Anesthesiologists (ASA) is a useful and widely accepted predictor for postoperative respiratory complications6. Eventually, a thorough physical examination is essential in order to detect any potential underlying pathology. Presence of abnormal chest auscultation, lower limb oedema and finger clubbing require investigation, when the underlying cause is unknown. Clinical evaluation tools predicting difficulties in intubation like the Mallampati classification and MACOCHA score should also be used6.
Imaging examinations
The necessity of preoperative chest imaging will most commonly be dictated by the physical examination and the patient’s medical history. The contribution of a preoperative chest radiograph in assessing the preoperative risk of respiratory complications is generally considered to be modest, given that the planned anaesthesia is rarely modified. Preoperative chest radiography should be performed in patients with known pre-existing cardiopulmonary disease (especially when there is a change in symptomatology), in patients aged >60 years who will be undergoing upper abdominal surgery, any thoracic surgery without any exceptions, and in abdominal aneurysm repair procedures, since an abnormal chest radiography is an independent predictor of postoperative pulmonary complications7.
Oxygenation assessment
Preoperative blood oxygen saturation via pulse oximetry (SpO2) is an easy, inexpensive and highly useful assessment of the patient’s cardiopulmonary functional status. SpO2 <90% has been shown to be associated with an increased risk of postoperative pulmonary complications8.
Measurement of blood gases determines the exact value of the partial pressure of oxygen (PaO2), but mainly specifies the value of carbon dioxide (PaCO2) and the patient’s acid-base status. Clear indications for the preoperative measurement of blood gases constitute low blood oxygen saturation measured with pulse oximetry, suspected acid-base disorder, HF, severe obstructive or restrictive respiratory disease irrespective of the cause, and the potentially high risk of postoperative pulmonary complications (increased possibility of requiring mechanical ventilation postoperatively), e.g. advanced age, and morbid obesity7,9.
Pulmonary function assessment
Spirometry and measurement of lung diffusing capacity have a clear role in the risk stratification of patients undergoing cardiothoracic surgery. For patients undergoing non-cardiothoracic surgery, although there are no data to support pulmonary function assessment as a routine test, this assessment is indicated in older patients (>60 years) and when relevant findings are identified in the patient’s medical history (Figure 1). Recent spirometry is needed in all patients with diagnosed respiratory disease, especially when there is a change in symptomatology, e.g. active or recent exacerbation in patients with COPD and asthma7.
Exercise tests
Restriction of the ability to exercise due to breathlessness is indicative of a patient’s cardiopulmonary status. When the patient is able to climb two levels of stairs or walk 350–400 m at a reasonable pace (5.5 km/h) without experiencing breathlessness, this exceeds 4 metabolic equivalents of energy expenditure, which are considered a safe threshold for elective surgery10. Lower levels of exercise are associated with an increased risk of respiratory and cardiovascular complications.
Ergospirometry is the method of choice for both the assessment of exercise capacity and the assessment of the contribution of the individual factors (respiratory, cardiovascular, musculoskeletal system) involved in exercise restriction. Ergospirometry is indicated in high-risk patients undergoing thoracic surgery (lobectomy, pneumonectomy). A maximum oxygen consumption (VO2max) of <10 mL/kg/min is associated with an increased risk of perioperative complications11.
Laboratory examinations
Among the laboratory examinations used to assess the risk of postoperative respiratory complications, low albumin (≤3.5 g/dL) is the strongest predictor. In fact, it is considered an equally powerful risk predictor as a significant patient-related risk factor7. Other laboratory examinations indicative of an increased risk of postoperative respiratory complications are increased urea nitrogen levels (≥30 mg/dL) and low haemoglobin levels7,8.
Risk indices for postoperative respiratory complications
Quantitative estimation of the risk of postoperative respiratory complications allows accurate prediction of complications and the design of a safe management plan (e.g. type of anaesthesia, invasiveness of the planned postoperative procedures, and setting of the postoperative follow-up). Multiple risk stratification indices have been developed for this purpose. Of those, the ARISCAT (Assess Respiratory Risk in Surgical Patients in Catalonia) and Gupta indices are validated and easily used in clinical practice12.
As per the ARISCAT risk index, patients are classified as low-risk (<26 points), intermediate-risk (26–44 points) and high-risk (≥45 points) risk based on 7 variables related to the patient and the surgical procedure to be followed (Table 1). The ARISCAT risk index predicts the risk of any postoperative respiratory complication, such as respiratory infection, respiratory insufficiency, bronchospasm, pleural effusion, pneumothorax, and aspiration pneumonia, especially in those patients undergoing thoracic or upper abdominal surgery13.
Table 1
ARISCAT (Assess Respiratory Risk in Surgical Patients in Catalonia) risk index*
* ARISCAT score calculation can be accessed for free at: https://www.mdcalc.com/ariscat-score-postoperative-pulmonary-complications#use-cases
In a validation study, the ARISCAT-predictive rates compared against the actual observed/measured postoperative respiratory complication rates in the low-, intermediate- and high-risk patients were 0.87% compared to 3.39%, 7.82% compared to 12.98%, and 38.13% compared to 38.01%, respectively, highlighting the reliability of this risk index14.
The Gupta surgical risk index predicts the risk of mechanical ventilation over 48 hours after surgery or the risk of unplanned intubation within the first 30 days after surgery12. As part of the Gupta risk index, the type of surgery, patient’s physical status according to ASA classification, emergency procedure, the patient’s functional status and the presence of sepsis are assessed. In each patient, the perioperative risk should be assessed in relation to the potential benefit of the planned surgical procedure. Surgery, as long as it is considered necessary, does not constitute an absolute contraindication even for patients with a severe underlying respiratory disease or at high risk of postoperative respiratory complications.
Disease-specific evaluation/management: Healthy smokers, patients with chronic obstructive pulmonary disease, asthma
Healthy smokers
Smoking is a public health problem, especially in Greece where approximately 1/3 of the Greek population are active smokers. Smoking delays tissue repair, which is essential for rapid recovery. It also increases in-hospital mortality by up to 20% and postoperative complications even in healthy smokers (pneumonia, coma, cardiac arrest, pulmonary embolism, sepsis and septic shock, prolonged stay in the intensive care unit) by 40%15.
Preoperative evaluation of an asymptomatic smoker should include spirometry (where the forced expiratory volume in one second/forced vital capacity [FEV1/FVC] ratio must be >70 or above the lower limit of normal [LLN]), evaluation of a coexisting phenotype of bronchitis or asthma, arterial blood oxygen saturation, and chest radiograph. Findings indicating restrictive functional impairment should lead to further investigation of the underlying cause. If SpO2 is normal, measurement of arterial blood gases is not necessary, except in cases of obesity, suspected sleep-disordered breathing (SDB) syndrome or when FVC <80%, since these factors have been shown to be independent risk factors for postoperative complications, even in ‘normal’ smokers16. The preoperative period, in anticipation of the upcoming surgery, provides the best opportunity to intensify smoking cessation strategies. Such strategies shall include:
Intensive behavioural therapy in a smoking cessation clinic, nicotine replacement therapy, and use of approved smoking cessation medications (varenicline, bupropion, nortriptyline, and cytosine) for at least 4–8 weeks. This approach not only leads to a 60% reduction in perioperative complications, but also helps to maintain abstinence from smoking after surgery17. Also, smoking cessation results in faster wound tissue healing, even after 4 weeks of smoking cessation. The role of a trained specialist in smoking cessation counselling constitutes the cornerstone in cessation success, with an impact seen even 6 months after surgery15.
Smoking cessation at least 8 weeks prior to surgery is associated with a 50% reduction in overall respiratory complications in former compared to active smokers.
The use of electronic cigarette as a means to facilitate faster smoking cessation has been proposed in the past. However, the multiple adverse health effects associated with electronic cigarette use raises concerns and is not indicated.
Patients with chronic obstructive pulmonary disease
COPD is the fourth leading cause of death worldwide. The preoperative evaluation of patients with COPD, as well as their management, should be based on the following main elements:
Assessment of the patient’s respiratory function using spirometry (estimation of FEV1, the FEV1/FVC ratio, and LLN) and where appropriate, especially in thoracic surgery, by calculating the diffusion lung capacity (DLCO). FEV1 values >80% are considered to be normal, with a low intraoperative risk of complications, while FEV1 values 50–80% and <50% are considered to confer a moderate and increased intraoperative and postoperative risk of complications, respectively16. Where lobectomy/pneumonectomy is required, the estimated postoperative FEV1 should also be determined; patients with a postoperative FEV1 <50% should also be assessed with a cardiopulmonary exercise test (CPET)18. DLCO values >60% are associated with a low risk of complications, while DLCO values <60% are associated with an increased rate of complications in patients with COPD and, therefore, CPET is recommended18.
Assessment of cardiopulmonary fatigue is of paramount importance in patients who will undergo thoracic surgery and have marginal respiratory function (FEV1 <50%, DLCO <60%). Generally, VO2max >10 mL/kg/min is associated with an acceptable rate of intraoperative complications18.
Clinical assessment of symptoms of the COPD patient, such as breathlessness, chronic cough and chronic sputum production. With the use of the modified Medical Research Council (mMRC) questionnaire, the degree of dyspnoea of the COPD patient can be precisely assessed, while the COPD Assessment Test (CAT) is used to assess the general health status of the patient. Both questionnaires are necessary for grading the risk of COPD into 4 stages (A, B, C, D), which is essential for identification of the proper treatment. Patients who do not receive optimal treatment (long-acting bronchodilators, inhaled corticosteroids, roflumilast) have suboptimal respiratory function, more disease exacerbations and a higher risk of perioperative and postoperative complications19.
The measurement of blood gases is essential in patients with COPD and low SpO2, severe COPD, dyspnoea, cyanosis, etc. Preoperative respiratory failure is associated with an increased rate of perioperative and postoperative complications19.
Successful preoperative preparation of COPD patients should also include a holistic therapeutic approach guided by a specialist physician, including enrolment of the patient in a pulmonary rehabilitation program, smoking cessation and nicotine replacement, influenza and pneumococcal disease vaccination, and dietary support (>10% of COPD patients suffer from malnutrition/cachexia). A 6-week pulmonary rehabilitation with twice-weekly supervised sessions, improved patients’ performance on the CAT and mMRC rating scales19. Also, proper dietary support can lead to an increase in fat-free mass and an improvement in nutritional markers (e.g. albumin), which are associated with fewer intraoperative and postoperative complications. Prior to urgently indicated surgeries, adjustment ant optimization of treatment should incorporate intense courses of short-acting bronchodilators, nebulised corticosteroids, and physiotherapy where applicable (i.e. in chronic bronchitis/bronchiectasis patients) along with smoking sessation16,19.
Patients with asthma
Globally, 4.3% of the population has asthma, while 8.4% of people in the western world are affected. The preoperative preparation of the asthmatic patient and staging of the severity and degree of symptom control according to international guidelines (e.g. Global Initiative for Asthma [GINA]) is of paramount importance20.
Preoperative evaluation of patients with asthma should include pre- and post-bronchodilator spirometry, and a full assessment of the degree of asthma symptom control20,21. The aim of optimising the treatment of the asthmatic patient is to achieve complete symptom control, minimise the frequency of exacerbations, and optimise the patient’s respiratory function 1–4 weeks before surgery21. At the same time, causes that could trigger an exacerbation (e.g. smoking, infections, allergens, exercise, and stress) should be avoided during the preoperative period. The majority of the studies report an excellent peri- and postoperative course of patients with well-controlled asthma, with cases of bronchospasm, asthmaticus status and difficult intubation to be rare. Prior to urgently indicated surgeries, adjustment and optimization of treatment should incorporate intense courses of short-acting b2 adrenergic agents, nebulised corticosteroids, along with steroids when applicable (i.e. severe/britle asthma patients, and chronic bronchitis/bronchiectasis patients) along with smoking sessation21.
Disease-specific evaluation/management: Obstructive sleep apnoea hypopnoea syndrome
The incidence of OSA is constantly rising, a fact attributed to the global obesity epidemic over the last decades22. Treatment of OSA involves a combination of general measures such as weight loss, smoking cessation, an increase in physical activity and usually the use of non-invasive positive airway pressure (PAP) ventilation devices, and/or intraoral devices23.
A significant part of the preoperative evaluation focuses on the diagnosis of cardiac and respiratory conditions, with limited emphasis on the diagnosis of any underlying SDB. However, the prevalence of OSA in surgical patients ranges between 6–17%24, up to 70% in obese patients or those with severe heart disease25,26, and a significant proportion of patients undergoing surgery have undiagnosed OSA, which in the majority of cases (60%) has not been diagnosed by attending physicians27.
OSA increases the likelihood of perioperative and postoperative complications 2- to 4-fold25,28. In addition, the apnoea-hypopnoea index is postoperatively increased in patients with OSA, and may also be increased in patients with preoperative subclinical SDB29. Most common complications concern the respiratory and circulatory systems25,30. The use of general anaesthesia and postoperative analgesia with opioids influences the pathophysiology of the syndrome and predisposes to complications. Opioid use reduces or eliminates the protective reflex of awakening in patients with OSA and increases upper airway resistance. Additional risk factors are obesity, diabetes mellitus, and cardiovascular disease. The association between the development of these complications and OSA severity remains unclear25. Figure 2 provides an algorithm for preoperative evaluation of patients with suspected or diagnosed SDB.
Figure 2
Algorithm for preoperative evaluation of patients with suspected or diagnosed sleepdisordered breathing
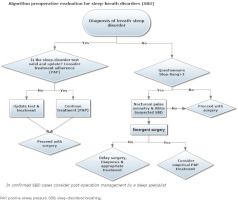
Recommendations for patients with high clinical suspicion of OSA
Obtaining a comprehensive medical history from the patient and his/her family and a targeted clinical examination can assist in raising the suspicion of the presence of OSA. Patients with excessive daytime sleepiness, snoring or witnessed episodes of apnoea during sleep are considered to have an increased probability of suffering from OSA31. Patients with obesity, cardiovascular comorbidities (arterial hypertension, HF, stroke, atrial fibrillation), type 2 diabetes mellitus and/or hypothyroidism also have an increased probability. Particular attention should be paid to patients undergoing bariatric surgery, as the prevalence of OSA in these patients can reach 94%32. An important predictor of OSA is the assessment of the upper airway for difficult intubation, including the Mallampati score.
Preoperatively, the use of specialised questionnaires is key for the detection of OSA. Screening questionnaires are characterised by high sensitivity but low specificity33,34, and thus high negative predictive value. This means that patients with low probability for OSA can be identified with a relatively high degree of accuracy. Patients with a high probability of OSA, as assessed by the questionnaires, should be further investigated.
The best-studied tool for the preoperative detection of OSA is the Stop-Bang questionnaire. This is an easy-to-use tool consisting of eight questions to which the patient is asked to answer ‘yes’ or ‘no’. The patient is classified as having ‘low’, ‘moderate’ or ‘high’ probability for the presence of OSA, with the last two categories associated with an increased risk of perioperative complications35. Although polysomnography is the gold standard, polygraphy or overnight oximetry can be used in certain cases for the preoperative assessment of OSA severity31.
Personalised management of patients with a high suspicion of OSA is of major importance, not only preoperatively, but also during the perioperative and postoperative period. Reduction of the surgical procedure duration and stress, selection of the appropriate type of anaesthesia, restriction of opioid use, transfer of the patient to the general ward in a state of complete alertness after surgery, use of an auto-CPAP device postoperatively and referral to a specialised sleep centre after hospital discharge are recommended. Finally, patients with a high suspicion of OSA appear to benefit from the preoperative application of PAP, when this option is available36,37.
Recommendations for patients with known, adequately controlled OSA
This category includes patients without residual symptoms associated with OSA, who display good compliance to treatment.
The assessment of patient compliance to and effectiveness of treatment should include a full sleep history and a record of any residual symptoms, recent sleep studies and the current therapy11,16. Ιn cases of doubt, the patient’s re-assessment with a new sleep study is recommended. In addition, patients who have undergone a surgical procedure for the treatment of OSA are considered to be at increased risk and a new sleep study is required preoperatively in case symptoms are reported.
Patients should be encouraged to continue their treatment (PAP device, intraoral devices) during hospital stay, and before and after surgery, since continuation of treatment has been associated with a decrease in cardiopulmonary complications36,37.
Recommendations for patients with known undertreated OSA
Patients with diagnosed but inadequately controlled OSA (patients with residual symptomatology and/or poor compliance to prescribed therapy) should be referred for a new sleep study and modification of therapy, especially when dysfunctions, such as hypoventilation, pulmonary hypertension or hypoxia are present25,28,36. These patients require intensive postoperative monitoring, since they experience increased postoperative morbidity and longer length of hospital stay25,28. As a result, preoperative clinical evaluation of the patient and monitoring for residual symptoms and for OSA treatment adequacy, are necessary.
The decision to either perform or postpone a surgical procedure depends on factors related to the surgical procedure itself and to the severity of the underlying OSA and of the patient’s comorbidities. The same applies to undiagnosed patients with a high clinical suspicion of OSA.
The management of patients with undertreated OSA is differentiated based on the severity of the surgery. Minor surgical procedures can be normally performed in most patients, however, treatment for OSA needs to be initiated postoperatively. With regard to major surgical procedures, if possible, surgery should be postponed and the patient should undergo a new evaluation and receive effective treatment for the underlying OSA. Finally, emergency surgical procedures should not be postponed due to OSA or suspected OSA, but should be performed with appropriate preoperative, perioperative and postoperative monitoring and management.
Disease-specific evaluation/management: Pulmonary hypertension and right-sided heart failure
Pulmonary hypertension (PH) and right-sided HF constitute important risk factors for perioperative morbidity and mortality. PH, regardless of the underlying aetiology, significantly increases perioperative mortality in patients with mild to moderate disease38. In a retrospective study of 18 million patients undergoing non-cardiac surgery, mortality was significantly higher in patients with comorbid PH (4.4% vs 1.1%)39. Mortality ranges between 2% and 10%, with the highest rate associated with emergency surgery40. Preoperative predictors of mortality in patients with PH are shown in Table 2 39.
Table 2
Factors associated with increased morbidity and mortality in patients with pulmonary hypertension
Common perioperative complications in patients with PH include: acute myocardial infarction, pulmonary embolism, cardiogenic shock41, cardiac arrhythmias, congestive HF, significant persistent hypoxaemia, respiratory failure, deterioration of renal function, and sepsis39. In addition, PH therapies (anticoagulants, prostanoids) increase the risk of bleeding.
General measures
General measures include assessment of the severity of PH and/or of the right-sided HF, of the patient’s functional status, and identification of modifiable factors, so as to optimise the patient’s condition. The anaesthesiologist in consultation with the surgeon and the treating physician (cardiologist or pulmonologist) should evaluate the patient’s condition and the necessity of the surgery, while non-surgical intervention should be sought in high-risk patients. The preoperative preparation of the patient could involve prolonged hospitalisation in order to optimise the patient’s endovascular volume, and hemodynamic and respiratory status.
Medical history and physical examination
The patient’s functional status, comorbid conditions (cardiovascular, pulmonary, renal, hepatic and haematologic) and symptoms with emphasis on easily-induced fatigue, dyspnoea, chest pain or syncopal episodes, and the patient’s New York Heart Association/World Health Organization classification status are assessed. The clinical examination includes the detection of signs of left- or right-sided HF. Such signs include: peripheral oedema, jugular distension, hepatojugular reflux, hepatosplenomegaly, ascites or systolic murmur of tricuspid regurgitation. In case of new onset or recent deterioration of these signs, a cardiac examination is required.
Preoperative evaluation
The necessary examinations required in patients with PH and/or HF preoperatively include: chest radiograph, arterial blood gases, electrocardiogram, echocardiogram, natriuretic peptide tests and stress test42. In patients receiving chronic treatment with diuretics, electrolyte imbalances that could cause cardiac arrhythmias (e.g. hypokalaemia) should be monitored and restored43. In case arrhythmias are detected during the preoperative evaluation, an electrophysiological study and, if necessary, pharmaceutical intervention or pacemaker placement should be performed. A significant proportion of patients with right-sided HF have an implantable pacemaker or cardioverter defibrillator. In these patients, the heart device should be checked prior to surgery44.
If hypoxaemia is detected through arterial blood gases, continuous oxygen therapy should be administered through an oxygen device, while if hypercapnia is detected, a sleep study should be performed, and non-invasive ventilation should be administered. OSA may contribute to hypoxaemia, which is an important cause of PH exacerbation. Therefore, in all patients nocturnal oximetry should be performed for detection of episodes of desaturation.
In case of a recent right heart catheterisation, the results should be provided to the anaesthesiologist in order to decide on the required perioperative interventions to be followed (mean central venous pressure, pulmonary arterial pressure, capillary wedge pressure, pulmonary vascular resistance, pulse volume, response to vasodilators and venous blood saturation).
Ensuring an optimum preoperative condition
The anaesthesiologist and the treating pulmonologist or cardiologist should ensure that the oxygenation, arterial pressure, heart rate and the pulsatile endovascular volume are optimal, chronic medication is continued uninterrupted, and factors that could cause an exacerbation of PH or right-sided HF have been addressed.
Management of chronic medication
Pulmonary arterial hypertension medication: oral, parenteral and inhaled formulations for pulmonary arterial hypertension should not be discontinued. These include prostacyclin agonists and analogues, endothelin receptor antagonists, nitric oxide and soluble guanylyl cyclase stimulators45.
HF medication: chronic medication of right- or left-sided HF such as beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor type 1 blockers, mineralocorticoid antagonists and digoxin should be continued. In certain patients, medication should not be administered within 12 hours prior to surgery to avoid intraoperative hypotension46.
Diuretics: chronic administration of diuretics is common in patients with PH and right-sided HF. Perioperative titration is guided by the haemodynamic parameters of the patient46.
Anticoagulants: the administration of anticoagulants should be adjusted based on the pharmaceutical formulation, the patient’s risk factors, and the bleeding risk of the surgery.
Optimisation of endovascular volume: patients with right-sided HF and/or PH have low tolerance to an increase in endovascular volume but also to a decrease in cardiac preload. Patients with fluid retention and hypervolaemia are expected to be aided by cautious administration of diuretics, so as to simultaneously avoid hypovolaemia. In patients who are likely to develop hemodynamic instability, close monitoring of haemodynamic parameters is required intraoperatively with central venous and pulmonary artery catheters, as well as with transesophageal ultrasound47.
Monitoring of exacerbation factors
Patients with PH should be monitored and treated with:
Oxygen therapy, for those with hypoxaemia.
Optimal treatment for the management of COPD exacerbation (e.g. bronchodilators, inhaled and systemic steroids, non-invasive ventilation for the treatment of hypercapnia, antibiotics in case of infectious exacerbation).
Treatment of comorbid OSA with CPAP.
Change in habits and lifestyle (smoking cessation, exercise, weight loss), provided sufficient time is available.
Anaesthesia agents
Mild preoperative sedation is useful in limiting increased sympathicotonia due to pain and anxiety. For this reason, sedatives, such as midazolam and opioids (fentanyl), should be administered in titrated doses. It is of utmost importance not to excessively sedate patients with PH, as this could lead to hypoventilation, deterioration of the existing hypoxaemia and/or hypercapnia, acute increase in pulmonary vascular resistance, and right ventricular dysfunction, which could lead to haemodynamic collapse. The effect of anaesthetic agents on right ventricular contractility and pulmonary vascular resistance, which should be taken into account by the anaesthesiologist are summarised in Supplementary file Table 1.
Disease-specific evaluation/management: Lung cancer/lung resection
Despite the introduction of numerous drug therapies during the last two decades, surgical resection remains the main method of treating early-stage non-small cell lung cancer. However, since the majority of lung cancer patients are active or former heavy smokers, surgical treatment may be associated with a high probability of perioperative complications and long-term disability due to impaired respiratory function. In addition, smoking also constitutes a risk factor for the development of atherosclerosis and coronary heart disease, further increasing the perioperative risk and contributing to further functional decline of patients undergoing surgical resection. Therefore, preoperative functional assessment of the respiratory and cardiovascular reserve is essential and should include the following.
Initial risk assessment by a multidisciplinary team
This team must include an oncologist, pulmonologist, radiotherapist and thoracic surgeon who will initially evaluate the risk-benefit ratio of the proposed radical surgical treatment compared to other options, which will then be communicated to the patient. The preoperative assessment should include: 1) the risk of perioperative death, 2) the risk of immediate serious perioperative complications, and 3) the risk of developing short- or long-term disability due to significant decrease of the respiratory functional reserve. Assessment of the risk of minimal invasive surgical procedures, if applicable, should also be performed in order to accurately determine the type of surgery and the extent of resection.
Cardiovascular risk assessment
Major cardiovascular complications after lung resection include acute myocardial infarction, severe arrhythmias (such as complete atrioventricular block and ventricular fibrillation) and sudden cardiac death, the incidence of which varies depending on the type of surgery and the patient’s risk factors. Based on the international guidelines issued by a wealth of scientific societies (American Heart Association/American College of Cardiology, European Society of Cardiology/European Society of Anaesthesiology, European Respiratory Society [ERS]/European Society of Thoracic Surgeons [ESTS]), assessment is initiated with detailed history taking, physical examination, electrocardiogram and assessment of the Revised Cardiac Risk Index (RCRI). This index enumerates the presence of 0, 1, 2, or ≥ 3 risk factors in the patient (Table 3). Patients with >2 risk factors or poor functional status (unable to climb 2 floors using the stairs due to cardiovascular symptoms) or suspected uncontrolled heart disease, require further specialised cardiac evaluation, based on a special algorithm (Figure 3A). Patients with 0–2 risk factors and a good functional status can proceed to further preoperative assessment with spirometry and CPET. It is recommended that the functional assessment of the cardiovascular system be comprehensive, especially for elderly patients48.
Table 3
Risk factors of the Revised Cardiac Risk Index (RCRI)*
Figure 3
Algorithm for the preoperative management of lung cancer patients who will undergo surgical resection, based on: A) international guidelines, and B) the writing team of the Hellenic Thoracic Society (HTS) guidelines
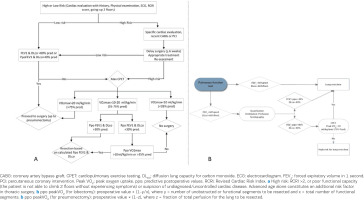
Of note, it is of major importance to highlight the fact that if a patient has undergone a drug eluting coronary stent placement, surgery should be postponed for up to 6 months. These patients are usually under double antiplatelet therapy. Delay of surgery, especially in high-risk for lung cancer patients with undiagnosed lung nodules might have detrimental effects.
Functional evaluation of the respiratory system
All patients should undergo preoperative assessment of FEV1 and DLCO, which are independent predictors of perioperative complications and postoperative mortality. In addition, the predicted postoperative (ppo) values of FEV1 and DLCO (ppoFEV1 and ppoDLCO) should also be calculated13. Based on ERS/ESTS and the American College of Chest Physicians (ACCP) guidelines, for patients undergoing lobectomy, ppo values are calculated by counting of the bronchopulmonary segments as follows: ppoFEV1 or ppoDLCO = preoperative value × (1-y/x), where y = number of unobstructed or functional segments to be resected and x = total number of functional segments.
Based on the above guidelines, for patients undergoing pneumonectomy, lung perfusion scintigraphy is required for the calculation of ppo values. The ppo values are calculated as follows: ppoFEV1 or ppoDLCO = preoperative value × (1-z) where z = fraction of total perfusion for the lung to be resected.
Further functional assessment of patients undergoing lung cancer surgical resection includes the performance of exercise tests, based on specialised perioperative risk scoring algorithms. Although simpler tests such as the 6-minute walk test have been used, CPET is significantly superior since it accurately assesses the functional cardiopulmonary reserve, and provides valuable prognostic information for perioperative risk grading. The algorithm for the preoperative assessment of lung cancer patients who will undergo resection based on international guidelines is presented in Figure 3A. The role of maximal CPET in the preoperative evaluation of lung cancer patients, based on international guidelines, is presented in Table 4. It should be mentioned, however, that the performance of CPET in lung cancer patients undergoing surgical resection who have FEV1 and/or DLCO <80% (i.e. the vast majority of the patients) is currently infeasible in the daily clinical practice in Greece. Therefore, a modified practical algorithm for the preoperative evaluation of these patients in the routine clinical practice is presented in Figure 3B, which constitutes a recommendation of the writing team of the Hellenic Thoracic Society (HTS) guidelines. This algorithm is not intended to replace international guidelines or recommendations but to support the clinical physician in the preoperative evaluation of cancer patients based on the examinations available in the routine care in Greece.
Table 4
Utility of CPET in the preoperative evaluation of lung cancer patients based on international guidelines
| Clinical decision | ACCP guidelines | ERS/ESTS guidelines | BTS guidelines |
|---|---|---|---|
| When is CPET recommended? | ppoFEV1 or ppoDLCO <30% predicted | FEV1 or DLCO <80% predicted | ppoFEV1 or ppoDLCO ≤40% predicted |
| Peak VO2 cut-off indicating low risk | >20 mL/kg/min (75% predicted) | >20 mL/kg/min (75% predicted) | >15 mL/kg/min |
| Peak VO2 cut-off indicating moderate risk | 10–20 mL/kg/min (35%−75% predicted) | 10–20 mL/kg/min (35%−75% predicted)a | |
| Peak VO2 cut-off indicating high risk | <10 mL/kg/min (35% predicted)b | <10 mL/kg/min (35% predicted)c | <15 mL/kg/min |
ACCP: American College of Chest Physicians. BTS: British Thoracic Society. CPET: cardiopulmonary exercise testing. ERS/ESTS: European Respiratory Society/European Society of Thoracic Surgeons. Peak VO2: peak oxygen uptake. ppoDLCO: predicted postoperative value of diffusing capacity of the lungs for carbon monoxide. ppoFEV1: predicted postoperative value of forced expiratory volume in 1 second.
a Resection is recommended up to a calculated extent according to ppoFEV1, ppoDLCO and ppo peak VO2.
c Lobectomy and pneumonectomy are not recommended. Adapted from Boutou et al.48 (2020).
Disease-specific evaluation/management: Perioperative evaluation of patients with interstitial lung disease
The most frequent and most serious postoperative complications affect the respiratory system and occur in 2% to 19% of the operated patients. In patients with interstitial lung disease (ILD), the likelihood of postoperative complications is high, with a mortality rate of 3% mainly due to the development of pneumonia or ILD exacerbation. The risk is higher in males, in patients with low body mass index (BMI <23 kg/m2), in the presence of dyspnoea, malignancy (especially lung cancer), and in patients with low FEV1 and FEV1/FVC. The longer duration of anaesthesia, emergency surgical procedures or procedures with potentially increased estimated blood loss, thoracic surgeries (including VATS lung biopsy and mediastinoscopy) are also aggravating surgery-related risk factors. Various algorithms have been developed for overall risk assessment, such as the aforementioned ARISCAT index (Table 1). Unfortunately, due to the fact that in clinical studies the number of patients with interstitial disease is not clearly defined, the ARISCAT index does not include important and distinct risk factors related to the unique pathophysiology of interstitial diseases8. Therefore, the Interstitial and Diffuse Lung Disease Network Steering Committee has proposed an approach for the evaluation of the preoperative risk that includes the ARISCAT index (Table 1), but also a set of patient- and surgery-related risk factors (Table 5)49.
Table 5
Risk factors for postoperative complications in patients with interstitial lung disease
| Patient-related risk factors |
| Male sex |
| Difussion lung capacity (DLCO) <60% predicted |
| Preoperative home oxygen requirement |
| Presence of acute exacerbation of interstitial lung disease |
| Pulmonary hypertensiona |
| Charlson Comorbidity Index score ≥2 |
| Immunosuppressed status |
| Obstructive sleep anpoeab |
| Surgery-related risk factors |
| General anaesthesia |
| Emergency surgery |
| Longer duration of anaesthesia/longer operative time (>2 h) |
| Pulmonary/thoracic surgery |
| Open rather than thoracoscopic surgery |
| Pneumonectomy or lobectomy (vs wedge resection)c |
a Transthoracic echocardiogram (right ventricular systolic pressure ≥40 mmHg) or right-sided heart catheterization with mean pulmonary arterial pressure >25 mmHg.
The contribution of each of the factors present in Table 5 to the evaluation of the postoperative risk cannot be appraised without prospective studies. However, the more risk factors a patient has, the higher the cumulative risk for any planned surgical procedure. Therefore, the following preoperative evaluation is recommended for all patients with ILD: spirometry, DLCO, 6-minute walk test, assessment of ASA class and Charlson’s Comorbidity Index, transthoracic echocardiogram, and screening for OSA6. The risks arising from the evaluation as per Tables 1 and 5 as well as from the specific characteristics and severity of the disease from which each patient suffers, should be taken into account and discussed with the patient49. Based on ARISCAT index and the evaluation of the additional risk factors in Table 5, the algorithm presented in Figure 4 is recommended for the evaluation of the postoperative risk in patients with ILD. Given the increased risk of complications in these patients, it is important to implement appropriate intraoperative measures to reduce mortality and morbidity50.
Figure 4
Algorithm for the preoperative evaluation of patients with interstitial lung diseases scheduled to undergo surgery
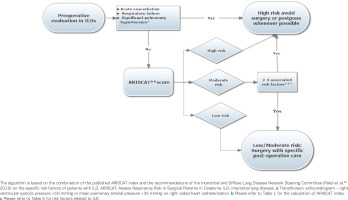
These measures include:
Avoid surgery when there are signs indicating infection;
Smoking cessation;
Avoid general anaesthesia, whenever possible;
Avoid neuromascular blockade, whenever possible;
Apply protective lung ventilation;
Administer intraoperative fluids cautiously;
Use high-flow nasal cannula after extubation;
Deep vein thrombosis prophylaxis; and
Early postoperative mobilization.
It is underlined that this algorithm is not validated by retrospective studies and cannot take into account the specific characteristics of patients with ILD. Notably, ILD represents a wide category of diseases with diverse pathophysiology, a fact that should be taken into account when deciding the surgical procedures to be performed and the anaesthesia to be administered.
CONCLUSION
We have outlined the disease-specific evaluation and management practices for patients with chronic obstructive pulmonary disease, asthma, obstructive sleep apnoea, pulmonary hypertension, right-sided heart failure, lung cancer, and interstitial lung disease, who are about to undergo thoracic surgery.