INTRODUCTION
Chronic obstructive pulmonary disease (COPD) generates functional limitations as a result of dyspnea, even on mild exertion, impairing functionality and quality of life1. Multiple investigations and organizations, such as the Initiative for Chronic Obstructive Lung Disease GOLD2, recommend prescribing physical exercise for pulmonary rehabilitation programs in patients with COPD. The dosage of training loads applied are based on the maximum performance thresholds attained after a cardiopulmonary exercise test (CPET)3,4.
At high altitude, understanding this as a geographical altitude above 2500 m and below 5500m, there is a decrease in the environmental partial pressure of oxygen (PO2)5. Exposure to hypobaric hypoxia in patients with COPD leads to increased dyspnea and further clinical deterioration6. Performing a CPET under these hypoxic conditions can lead to biased final results that would affect the prescription of the exercise7. Thus, since around 10% of known COPD cases worldwide live at high altitude6, it is essential to design an adequate CPET for these patients. As a preliminary approach, here we present a case report of a COPD patient living at high altitude who performed three different CPET protocols until achieving its maximum threshold.
This case report was prepared to consider the indications of The CARE case report guidelines that should be followed (Supplementary file).This research was carried out following the principles of the Declaration of Helsinki and was endorsed by the institutional ethics committee of the Faculty of Sciences of the Universidad Nacional de Colombia granted on 7 December 2020, and registered with approval number 14-2020. The participant signed an informed consent agreeing to her participation.
CASE PRESENTATION
Our patient was a 66-year-old woman diagnosed with COPD GOLD1B, without pulmonary hypertension (mean pressure in pulmonary arteries of 21 mmHg), and respiratory exacerbations during the 6 weeks before the measurements, with a record of cigarette consumption for 43 years with 38 pack-years, but currently a non-smoker (23 years ago). She has a history of pharmacologically controlled hypothyroidism and type II diabetes, without a medical history of neurological, hematological diseases, cardiovascular diseases, or sleep disorders that would affect arterial saturation measurements. No disabilities were found that limited the execution of the CPET.
The patient presented arterial desaturation at rest (SpO2 84%) without requiring oxygen therapy and without signs of respiratory distress (Table 1). The patient reported a score of 17 on the COPD assessment test (CAT, 2009) and dyspnea with score 3/4 on the Medical Research Council Dyspnoea Scale (mMRC, 1959). She also reported a low level of physical activity presenting 1.2 METs/hour and more than 3000 steps/day, measured by accelerometry for 7 days with Triaxial ActiGraph GT3X+ (Pensacola, USA).
Table 1
Patient characteristics and CPET protocols
| Characteristics | CPET protocols | |||||
|---|---|---|---|---|---|---|
| Timeline | Phase | Time (min) | Workload (W) | Speed (rpm) | ||
| Sex | Female | First measurement (M1)8 | Rest | 2 | 0 | 0 |
| Age (years) | 66 | Warm-up | 3 | 0 | 60 | |
| Height (cm) | 160 | Start of load | 2 | 25 | 60 | |
| Weight (kg) | 87.5 | Load increase | Every 3 | 25 | 60 | |
| BMI (kg/m2) | 33.6 | Active recovery | 2 | 10 | 30 | |
| Physical activity level | Passive recovery | 2 | No specified | |||
| METs/hour | 1.04 | |||||
| Steps/day | 2037 | Second measurement (M2) | Rest | 2 | 0 | 0 |
| Pulmonary function | Warm-up | 3 | 15 | 15 | ||
| FVC (%) | 70 | Start of load | 2 | 20 | 40 | |
| FEV1 (%) | 78 | Load increase | Every 2 | 10 | 60 | |
| FEV1/FVC (%) | 65.5 | Active recovery | 2 | 10 | 30 | |
| PEF (L/S) | 5.7 | Passive recovery | 2 | No specified | ||
| Other | ||||||
| mMRC | 3/4 | Third measurement (M3) | Rest | 1 | 0 | 0 |
| CAT | 17 | Warm-up | 2 | 0 | 30 | |
| Vital signs - baseline | Start of load | 2 | 30 | 60 | ||
| SpO2 (%) | 84 | Load increase | Every 2 | 15 | 60 | |
| HR (bpm) | 87 | Active recovery | 2 | 0 | 40 | |
| BP (mmHg) | 120/86 | Passive recovery | 5 | Sitting, without pedaling and quantifying the HR after 1, 3 and 5 min | ||
| BF (breaths/min) | 16 | |||||
[i] Environmental variables during measurement: altitude 2640 m, barometric pressure 752.1 hPa, temperature 14°C, humidity 62%. BF: breathing frequency. BMI: body mass index. BP: blood pressure. CAT: COPD assessment test. CPET: cardiopulmonary exercise tests. FEF: mean expiratory flow. FEV1: forced expiratory volume in 1 s. FVC: forced vital capacity. FEV1/FVC: forced expiratory volume in 1 s /forced vital capacity. mMRC: Medical Research Council Dyspnea Scale. HR: heart rate. METs: metabolic equivalents. PEF: peak expiratory flow. rpm: revolutions per minute. SpO2: pulse oxygen saturation.
Environmental variables during measurement
The cardiopulmonary physical exercise test in the patient with COPD was developed under the following geographical and environmental conditions: 1) region Andean, 2) altitude 2640 m, 3) barometric pressure during measurement 752.1 hPa, 4) temperature 14°C, 5) humidity 62%, and 6) Measurement time 10:00 to 10:30 a.m.
Cardiopulmonary exercise test
For the recognition of the physical condition, the CPET protocol presented by Schneider et al.8, was followed, which is a protocol indicated and developed for people with COPD. The first measurement (M1) was achieved according to what was postulated by the author (Table 1) using the Velotron DynaFit Pro-RacerMate cycle ergometer (Seattle, USA) accompanied by the Cosmed Quark-B2 gas analysis system (Rome, Italy).
After performing the M1, we found that the test did not meet the minimum time required according to the American Thoracic Society (ATS) (Statement on Cardiopulmonary Exercise Testing)9. Therefore, two new measurements (M2 and M3) were defined by adjusting workloads, rpm, and times of each phase (Table 1), until the ATS criteria were achieved.
The patient performed each test with a 2-week difference to avoid distorting the results due to cumulative fatigue. The following variables were taken into account as maximum result variables of the CPET: duration of the test (time in minutes); peak oxygen consumption (V̇ O2peak in mL/kg/min); respiratory quotient (RQ); maximum heart rate at the end of the CPET (final HR); recovery heart rates at 1, 3 and 5 minutes after the end of the test; ventilatory O2 and CO2 equivalents (V̇ E/V̇ O2, V̇ E/V̇ CO2,); and volume of oxygen consumed (V̇ O2) and produced volume of CO2 (V̇ CO2).
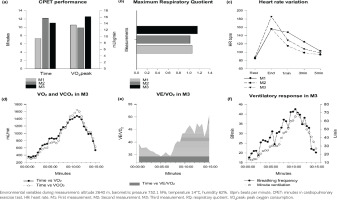
In the first protocol (M1), we recorded a 19% higher HR, 31% higher BF, and a 5% lower RQ in a shorter test time (6 min), than the results presented by Schneider et al.8. Values of V̇ E/V̇ O2 of 34.5 and V̇ E/V̇ CO2 of 35.3, were recorded without reaching the anaerobic threshold (AT), lactate 1.6 mmol/L, and RQ of 1.06. In M1, the time on the test did not meet ATS recommendations, as a successful CPET test should last at least 8–12 minutes, with reported RQs >1.10 (Figures 1a and 1b)9.
Recognizing that as COPD mitochondrial degradation increases, the proportionality of oxidative fibers decreases, muscle capillarization is reduced, and the hypobaric hypoxia in COPD increases the intensity and frequency of bronchospasm, decreases ventilatory muscle capacity, decreases fatigue tolerance and reduction physical fitness, for the second protocol (M2) we decided reduce the warm-up time, the initial load to 20 W, and the load increased to 10 W every 2 minutes with a 40 rpm. In this way, the initial load was reduced to optimize the test time and the values of HR, RQ, and V̇ O2peak according to the recommendations of ATS. We found that although the test time increased by 35%, the RQ, V̇ O2peak, HR were lower than in M1, and a V̇ E/V̇ O2 of 34.6 and V̇ E/V̇ CO2 of 35.2 were found without AT achievement, lactate 1.4 mmol/L and RQ of 1.02 (Figures 1a and 1b).
In the third protocol (M3) we reduced the heating speed (rpm), maintaining the same time, increasing the initial load by 35%, increasing 15 W every 2 min, and the speed (rpm) by 35%, to achieve the AT in a shorter testing time. After this protocol we found: 1) an optimal test time without counting the warm-up; 2) a final HR 16% higher than the previous tests; 3) an RQ of 1.16, which is 9% greater than that recorded in M1; 4) a 16% increase in the V̇ O2peak recorded in the first determinations; 5) an increase in the levels of ventilatory response (Figures 1d–1f); and 6) the highest record of V̇ E/V̇ O2 of 36.4 and V̇ E/V̇ CO2 of 37.3 with lactate 2.4 mmol/L, and RQ of 1.16. Finally, we consider important to specify the criteria for the recovery phase when adjusting the protocol, since the scientific literature does not provide detailed information on this phase.
DISCUSSION
Based on the preliminary results derived from the case reported here, the standardized protocol M1 proposed for patients with COPD GOLD1 is not the best CPET for patients residing at high altitudes. In these patients, dyspnea considerably increases with physical exertion because of the effect that the environmental hypoxia (low PO2) has on the patients’ cardiorespiratory function.
The results found in this case report lead us to think that a CPET protocol for people with COPD exposed to high altitude could be the incremental protocol that was executed in M3, which consists of: one minute of measurement at rest on the cycle ergometer, two minutes of warm-up at 30 rpm without load, start of the test with a pedaling at 60 rpm for 2 minutes with a load of 30 W, and increasing the load by 15 W every 2 minutes until fatigue, two minutes of active recovery, pedaling without load at 40 rpm, and 4 minutes of passive recovery in a sitting position.
The decreased tolerance to fatigue and performance of CPET in people with COPD at high altitudes may be related to the effects of hypobaric hypoxia on increased bronchospasm and the acute inflammatory response10, the decreased efficiency of the ventilatory muscles, the increase in physiological dead space, and the exacerbation of pulmonary hypertension11. Considering that low PO2 environments exacerbate the clinical condition of people with COPD, it is advisable to adjust CPET protocols for highaltitude environments. It can be considered that a CPET test that does not adjust to the environmental conditions can lead to biased results regarding the physical condition of the people; which would affect pulmonary rehabilitation programs based on prescribed physical exercise7.
All healthcare professionals working with respiratory patients must search for the most effective and forceful intervention strategies and adapt them to the real state of the patients to favor the pulmonary rehabilitation process in patients with diagnoses of respiratory diseases and deterioration of the health condition due to a sedentary lifestyle. Considering that altitudinal hypoxia deteriorates the global function of patients with COPD and that pulmonary rehabilitation programs are based on physical exercise programs, we consider necessary to recognize which CPET protocol is more convenient and efficient for people with COPD exposed to altitudinal hypoxia. Knowledge of the maximum thresholds of physical fitness is necessary to establish physical training programs according to the conditions of each patient. We recognize the need to stipulate CPET protocols that accommodate the hypobaric hypoxia present in high-altitude geographical regions. Finally, we believe that the results obtained could be taken as a starting point to develop CPET tests in COPD at high altitude.