INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is transmitted through droplets, attaches to the angiotensin converting enzyme-II (ACE-II) receptors on the surface of the cells via the spike protein and enters the cell. ACE-II receptors are mostly found in the respiratory tract and lungs. Coronavirus disease 2019 (COVID-19) is primarily a lung disease, although it affects all organs. An important part of the symptoms (such as cough, shortness of breath) is associated with respiratory tract involvement. A spike in inflammatory mediators may explain lung tissue damage in COVID-19 patients, known as the ‘cytokine storm’1. The use of corticosteroids may help control this cytokine storm, alleviating tissue damage and ultimately fibrosis2. However, acute respiratory distress syndrome (ARDS) is the leading cause of mortality in COVID-19 pneumonia3. Despite the evidence for amelioration of secondary ARDS4, the efficacy of glucocorticoids on ARDS secondary to respiratory distress and viral infections is controversial for SARS-CoV-1 and MERS-CoV5,6. However, in recent meta-analyses, it has been published that glucocorticoids reduce mortality in the treatment of ARDS due to COVID-197,8.
Since the effects of COVID-19 are mostly on the lungs, hypoxia and accordingly shortness of breath and increased respiratory rate are the most important clinical conditions in patients with diffuse lung involvement. In patients with moderate and severe pneumonia, a decrease in oxygen saturation is often observed in the follow-up. While many patients recover without sequelae in terms of the lung, some patients experience loss of function manifested by exertional dyspnea9.
The aim of our study was to investigate the long-term effects of different doses of steroid therapy given in the acute period on pulmonary function and radiological findings in hospitalized COVID-19 patients.
METHODS
Participants and study design
This single-center, prospective, observational cohort study was performed on hospitalized COVID-19 patients in the period April 2020 to July 2021. The study population consisted of adults aged 18–65 years who were admitted to our hospital, who were confirmed positive for COVID-19 by PCR in a nasopharyngeal swab. Patients aged ≥65 years were excluded from the study in order to exclude deterioration in pulmonary functions due to physiological changes brought on by aging10. The inclusion criteria were: being hospitalized with COVID-19 pneumonia, having passed at least 3 months after discharge, and aged 18–65 years. The exclusion criteria were: being diagnosed with chronic lung diseases, having a neurological, cardiovascular, and musculoskeletal conditions that could prevent performing the assessments, having a previous history of lung transplantation, being treated with long-term oxygen therapy, having an active infection, being pregnant, and aged ≥65 years.
All treatment regimens for COVID-19 were implemented based on the COVID-19 Guidelines of the Turkish Ministry of Health. These guidelines are regularly revised and updated based on scientific advances in the treatment of COVID-19. Therefore, the treatment modalities of patients may differ according to the currently valid version of the guidelines at the time the patient was diagnosed with COVID-19.
Steroid treatment and dosage were determined by three pulmonologists based on these guidelines. Patient data were obtained from survivors. The data of 671 patients treated in the hospital were scanned from our hospital system.
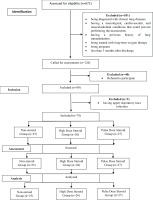
In all, 120 patients who met the inclusion criteria were informed and invited; 80 patients who applied to the study after the invitation were evaluated. Two of the 80 patients were excluded from the study due to newly developing upper respiratory tract infection (Figure 1).
The patients were divided into 3 arms according to the steroid doses they received. Those who received 250 mg methylprednisolone treatment for at least 3 days were considered to be in the pulse-steroid group. Those who received 40 mg methylprednisolone treatment for at least 3 days were considered as high-dose steroid group. In the other arm, there were patients who did not receive any steroid treatment. In addition, all patients received standard favipravir treatment for 5 days. Apart from this, all patients received supportive treatment during hospital follow-up.
Data collection
The authors reviewed electronic medical records and extracted data for the period between admission to discharge, death, or 15 July 2021, whichever occurred first. Demographic and clinical characteristics, and radiological and laboratory information, were recorded including comorbidities, respiratory variables, and details of treatments administered for COVID-19. In addition, the average hospital length of stay (days) and the time at which the patients were called for assessments (home-time after discharge in months) of all three groups were recorded. The measurements of spirometry, diffusing capacity of the lungs for carbon monoxide (DLCO), plethysmography, 6-minute walk test (6MWT), the modified Borg dyspnea scale, and thorax computed tomography (thorax CT) were all done on the same day and included in the analysis.
Outcome measures
The tests of spirometry and body plethysmography were performed (Vyntus, body plethysmograph, CareFusion, Germany) using European and American Thoracic Society guidelines11. The percentage of predicted values of spirometry was calculated according to GLI (Global Lung Function Initiative) 2012 reference standards12. The DLCO was determined by the single-breath technique, in accordance with the ERS/ATS guideline13. The percentage of predicted values of DLCO was calculated according to GLI reference values14. The 6MWT was performed in accordance with the guideline of ATS15. Percentage of predicted values of the 6MWT was calculated according to the 6-minute walk distance in healthy patients16. The walking distance in 6 min was recorded. Oxygen saturation and heart rate were also measured before and after the test using a pulse oximeter. (Beuer oximeter; Beurer GmbH; Germany). Dyspnea was rated on the Modified Borg Scale. The anchors were ‘0’ for no dyspnea and ‘10’ for maximum dyspnea17. Thorax CT images were taken in the supine position, during the inspiration phase. The draft parameters were 100–120 kV tube voltage, 50–399 mA, and 1.5 mm section thickness. The standardized uptake value, ground-glass opacity (>10% area), lesion density (presence of pure ground-glass opacities (GGO), ground-glass opacities with consolidation, consolidation), linear densities (interlobular septal line, intralobular septal line, parenchymal bands), crazy paving, pleural effusion, atelectasis and pulmonary fibrosis were evaluated with thorax CT. The presence, extent, and distribution of interstitial findings were recorded using the terminology recommended by the Fleischner Society18. The presence of GGO, consolidation, interstitial thickening, and fibrosis were quantitatively analyzed using a radiological scoring system ranging 0–25 points that had previously been used to define idiopathic pulmonary fibrosis caused by SARS19-21. Each of the 5 lung lobes was evaluated between 0–5 points on the basis of the relevant area. Each lobe was evaluated according to the area of ground glass opacity, and 0 point was given for normal performance, 1 point was given if the area contains <5% of the lobe, 2 points were given if the area contains <25% of the lobe, 3 points were given if the area contains 25–49% of the lobe, 4 points were given if the area contains 50–75% of the lobe, and 5 points were given if the area contains >75% of the area. Individual segment scores were aggregated as total scores in statistical analysis. Thorax CTs of all patients were evaluated together by the same radiology specialist and chest diseases specialist.
Statistical analysis
Statistical analysis was conducted using IBM SPSS v.26 (SPSS Inc., USA). The normality of the distribution of data was analyzed using Shapiro-Wilk test. The analysis of covariance (ANCOVA), adjusted for the home-time after discharge and the oxygen therapy, was used to compare values of spirometry, diffusion capacity, plethysmography, CT score and 6MWT between all three groups. Multiple comparison tests were performed to detect the differences among the groups using Bonferroni post hoc test corrected significance. In addition, the effect size estimates were calculated by partial eta squared (ηp 2). Categorical variables were compared between groups using chi-squared test. The results were considered significant at p<0.05.
RESULTS
In the study, 78 patients were evaluated: 25 patients who did not receive steroid therapy, 26 patients who received high doses, and 27 patients who received pulse doses.
The demographic and clinical characteristics of participants are given in Table 1.
Table 1
Demographic and clinical characteristics of patients
There were no significant differences in the demographic and clinical characteristics of all the groups (p>0.05). There were significant differences between the home-time after discharge and the oxygen therapy of all groups (p<0.001).
The comparison of the values of spirometry, diffusion capacity, plethysmography, CT score and 6-minute walk test, with the ANCOVA test adjusted for the home-time after discharge and the oxygen therapy, among all groups are given in Table 2. The values of spirometry did not show a statistically significant difference among all groups except peak expiratory flow, PEF (% pred) (p=0.044; ηp 2=0.082). The value of PEF (% pred) was significantly lower in the pulse-dose steroid group compared to the high-dose steroid group (p=0.040). There was a significant difference in the values of DLCO (% pred) and DLCO/VA (% pred) among all groups (p=0.027, ηp 2=0.094; p=0.048, ηp 2=0.080, respectively). Both the values of DLCO (% pred) and DLCO/VA (% pred) were significantly lower in the pulse-dose steroid group compared to the high-dose steroid group (p=0.022, p=0.049, respectively). The CT score was significantly higher in pulse-dose steroid group compared to both the non-steroid group and high-dose steroid group (ηp 2=0.087; p=0.027, p=0.043, respectively). The 6MWT (% pred) was significantly lower in the non-steroid group compared to both the high-dose steroid group and pulse-dose steroid group (ηp 2=0.112; p=0.015, p=0.048, respectively).
Table 2
Comparison of the values of spirometry, diffusion capacity, plethysmography, CT score and 6-minute walk test among all groups
[i] FEV1: forced expiratory volume in 1 s. FVC: forced vital capacity. PEF: peak expiratory flow. FEF25: forced mid-expiratory flow at 25%. FEF50: forced mid-expiratory flow at 50%. FEF75: forced mid-expiratory flow at 75%. DLCO: diffusing capacity for carbon monoxide. DLCO/VA: DLCO divided by alveolar volume. TLC: total lung capacity. RV: residual volume. FRC: functional residual capacity. CT: computed tomography. 6MWT: 6-min walking test. MBS: Modified Borg Scale.
DLCO (% pred) was <80% in 51.3% of patients. DLCO/VA (% pred) was <80% in 6.6% of patients. In addition, 18.4% total lung capacity (TLC) (pred%) <80%, 14.5% residual volume (RV)/TLC (% pred) >120%, 32.1% PEF (% pred) <80%, 10.3% forced expiratory volume in 1 s (FEV1) (% pred) <80%, 9% force vital capacity (FVC) (% pred) <80% were detected. The 6 MWT (%pred) of all patients was >80%.
The most common thorax CT abnormalities detected were reticulations (64.4%) and ground glass opacity (55.6%). Thorax CT of 13.3% of the patients had completely recovered. Thorax CT involvement score was between 0–4 in 48.9% of our patients, whose mean score of thorax CT involvement was 6.47.
DISCUSSION
To our knowledge, this is one of the few studies investigating the long-term effects of different doses of steroid therapy on pulmonary function and functional capacity in hospitalized patients with COVID-19 pneumonia. Our study demonstrates that pulse-dose steroid may have a negative effect on the recovery term of pulmonary function in patients with hospitalized COVID-19 pneumonia. The values of PEF (% pred), DLCO (% pred), and DLCO/VA (% pred) in the pulse-dose steroid group were lower compared to the high-dose steroid group. The value of CT score (% pred) in the pulse-dose steroid group was higher than both the high-dose steroid group and the non-steroid group. However, the patients with the best pulmonary function were those who received high-dose steroid therapy. This treatment, which may have effects on mortality in the acute period7, may also positively affect pulmonary function in the long term.
According to previous experience with coronavirus pulmonary involvement of SARS and MERS, radiological abnormalities, pulmonary dysfunction, and decreased exercise capacity improve over time, but may persist for months or even years in some patients22–24. Although these data suggest that some patients will have long-term respiratory complications, the outcome of COVID-19 pneumonia in those recovering from acute infection is uncertain, despite some studies up to 6–12 months after discharge25-29.
However, until now, the effect of steroids on the long-term complications of COVID-19 has not been fully elucidated because most of the studies have short-term primary endpoints on mortality30,31. Steroids may reduce lung injury and fibrosis by decreasing the expression of pro-inflammatory mediators in lung tissue, including TNF-a, IL-1a, IL-1b, IL-6 and IL-12 p4032. Steroids are thought to be recommended because of their effect on immune-mediated lung injury and downregulation of the cytokine storm33.
The study by Baros et al.34 found that 0.5 mg/kg methylprednisolone treatment given during the acute phase in hospitalized COVID-19 patients reduced the deterioration in pulmonary function, FEV1 and FVC parameters, on the 120th day. In this study, pulse steroid effects, DLCO and radiology effects were not investigated. In our study, we did not find any difference between the 3 groups in terms of these parameters. However, important parameters such as PEF, DLCO, DLCO/VA, and 6 MWT were more preserved in patients receiving high-dose steroids. Since impaired diffusion is indicative of ongoing damage and possible fibrosis in the alveolar-capillary space35, our findings are remarkable and may be due to the previously mentioned positive effects of steroids. Similarly, 6DYT and PEF values may be better in this group due to a milder effect of the disease or less steroid myopathy.
In some systematic meta-analyses, although there are results showing that steroid treatments reduce mortality in the acute period in COVID-19 pneumonia, it has been found that
Pulse-dose steroid therapy does not reduce mortality compared to low-high-dose steroid therapy36,37.
A recent meta-analysis emphasized that systemic steroid use may be a potential benefit in the context of COVID-19 pneumonia, with studies showing that higher steroid dosages carry a higher risk38. Our findings show that pulse-dose steroid therapy in the acute phase does not have a positive effect on pulmonary function in the long-term. Based on a previous study, pulse doses may have decreased ARDS recovery and increased sequelae39. However, this finding may be related to the administration of steroids late in ARDS and the persistence of diffuse alveolar damage33.
According to a meta-analysis to evaluate long-term functional effects, the most important parameter is lung diffusion capacity. An important limitation is that the previous pulmonary functions of the patients were not known in the studies, and at least 3-month spirometry evaluation was recommended in patients with a potential for pulmonary sequels40. To minimize this limitation in our study, we excluded potential diseases and conditions affecting the lung. In our study, the most common impaired parameter was DLCO (% pred) in 51.3% of our patients. In addition, a decrease of 6.6% DLCO/VA (% pred) was detected. The rate of patients with FVC loss (9%) shows the importance of DLCO in the evaluation of lung function loss and that spirometry alone is insufficient.
Finally, we found discordant results between the groups in terms of CT abnormalities and lung function. Baros et al.34 associated this with functional adaptation and recovery. The discordance between gas-blood exchange abnormalities and radiological findings suggests that different mechanisms may underlie these changes. In addition to interstitial abnormalities, pulmonary vascular abnormalities may also have effects26.
Limitations
Our study has some limitations. The first limitation of the present study is it was single-center and had a limited number of patients. The second one is the previous pulmonary function of the patients was unknown. Third limitation of the study is that although it is a methodologically revealed situation and analyses were performed with the appropriate statistical method in a way to minimize the effects on the results, the baseline difference in ‘home-time after discharge’ and ‘oxygen therapy’ between the steroid groups may have affected our findings and interpretations. Future studies with comparisons without baseline difference in ‘home-time after discharge’ and ‘oxygen therapy’ between the groups are needed to contribute to the literature. On the other hand, the fact that chronic diseases affecting pulmonary functions were not included in the study and the patient group was young, are among the strengths of our study in evaluating the effects of COVID-19 pneumonia and steroid therapy on the lungs. As we mentioned, pulmonary function effects in patients receiving pulse steroid may be due to the severity of the disease. Due to the lack of treatment and vaccine options, especially in the early stages of the pandemic, some patients will need to be re-evaluated in terms of long-term sequelae. In addition, the level of sequelae may be more advanced in patients with chronic disease and in the elderly. For these reasons, future studies are needed.
CONCLUSIONS
Our findings show that pulse-dose steroid therapy given in the acute period of COVID-19 pneumonia may have negative effects on pulmonary function and recovery in the long-term, and that high-dose steroid therapy may have a positive effect on pulmonary function compared to pulse-steroid therapy and non-steroidal therapy. Patients who are given pulse-dose steroid during the hospital follow-up should be followed up for a longer time and closely.