INTRODUCTION
Although prone ventilation is widely used in acute respiratory distress syndrome (ARDS)1, its effects on right heart function assessed by state-of-the-art echocardiography are not well studied. Other investigators and we recently showed that transthoracic echocardiography may be feasible in intubated patients undergoing prone ventilation2,3. Accordingly, using two-dimensional transthoracic speckle tracking echocardiography, we investigated the effect of prone ventilation on right ventricular free wall strain (RVFWS)3. Here, we report the effect of prone ventilation on right atrial strain in a patient with severe ARDS, after optimization of lung recruitment and hemodynamics using esophageal pressure and pulmonary artery catheter measurements, respectively.
CASE PRESENTATION
We present the case of a 66-year-old obese (body mass index of 32.7 kg/m2) Caucasian man who underwent prone ventilation due to ARDS. Seven days before his intubation, the patient developed fever up to 38.5oC and vomiting. At that time, he was tested positive with a Severe Acute Respiratory Syndrome Coronavirus-2 nucleic acid assay. Two days before his intubation, the patient presented in the emergency department with dyspnea. His medical history was notable for left heart failure of ischemic etiology (coronary artery disease with coronary artery bypass graft 14 years ago), dyslipidemia, diabetes mellitus and chronic obstructive pulmonary disease. His medications included metoprolol, amlodipine/valsartan, acetylsalicylic acid, ezetimibe/simvastatin, vildagliptin/metformin, glibenclamide, and inhaled fluticasone/vilanterol. He had received his vaccination [a single-dose Ad26.COV2.S (Johnson & Johnson) vaccine] five months ago. On examination, the patient appeared alert and oriented to time, place, and person. The temperature was 37.5°C, the blood pressure 150/70 mm Hg, the heart rate 80 beats per minute, his breathing labored and the oxygen saturation was 89% at rest while breathing ambient air. There was no peripheral edema. Chest radiography revealed bilateral ground glass opacities. His laboratory findings were unremarkable. The patient was admitted to the hospital and received prophylactic enoxaparin, remdesivir, dexamethasone, ceftriaxone and moxifloxacin on top of his regular medications. During his hospitalization in the ward, his hypoxemia worsened and after a failed trial of high flow nasal oxygen, the patient was intubated4. He was admitted to the intensive care unit, while undergoing protective lung ventilation and meeting criteria of severe ARDS.
Within 12 hours following the intubation of the patient, an esophageal balloon was placed to optimize lung recruitment; external positive end-expiratory pressure (PEEP) was accordingly titrated to 15 cmH2O to maintain a positive transpulmonary pressure at end-expiration. Also, two-dimensional transthoracic speckle tracking echocardiography was performed and a pulmonary artery catheter was placed to optimize hemodynamics. The patient was then placed in prone position for 18 consecutive hours with improvement in terms of arterial blood gases and lung recruitment (allowing for a decrease in external PEEP to 12 cmH2O) and then he was repositioned supine. Table 1 depicts ventilatory settings and pulmonary parameters, while Table 2 depicts hemodynamic and advanced echocardiographic parameters. The abovementioned parameters were measured or calculated at four time points; namely, at baseline (immediately before prone positioning), one hour and 18 hours after prone positioning (i.e. while the patient was undergoing prone ventilation), and one hour after supine repositioning to allow for stabilization of hemodynamic changes induced by the postural change5.
Table 1
Ventilatory settings and pulmonary parameters before, during and after prone ventilation
[i] PEEP: positive end-expiratory pressure. PaO2: partial pressure of arterial oxygen. FiO2: fraction of inspired oxygen. PaCO2: partial pressure of arterial carbon dioxide. During the study period, the patient was undergoing volume-controlled mechanical ventilation and was under continuous infusion of cisatracurium.
Table 2
Hemodynamic and advanced echocardiographic parameters before, during and after prone ventilation
| Parameters | Supine at baseline | Prone at one hour | Prone at 18 hours | Supine repositioning |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 107 | 91 | 108 | 92 |
| Heart rate (bpm) | 72 | 64 | 78 | 66 |
| Norepinephrine intake (mcg/kg/min)* | 0.24 | 0.21 | 0.16 | 0.16 |
| Lactate (mmol/L) | 1.4 | 1.2 | 1.1 | 1.1 |
| Fluid balance from baseline (mL) | - | 0 | +380 | +410 |
| Right atrial pressure (mmHg)** | 12 | 10 | 10 | 7 |
| Pulmonary artery pressure, mean (systolic/diastolic) (mmHg)** | 30 (45/21) | 26 (37/18) | 27 (39/20) | 18 (33/11) |
| Pulmonary artery occlusion pressure (mmHg) | 26 | 13 | 14 | 8 |
| SvO2 (%) | 65 | 59 | 75 | 73 |
| Left atrial strain (%) | 16 | 18 | 24 | 26 |
| TAPSE (mm) | 7 | 13 | 13 | 14 |
| RVFWS (%)§ | 9 | 12 | 15 | 22 |
| Right atrial strain (%)† | 6 | 14 | 19 | 26 |
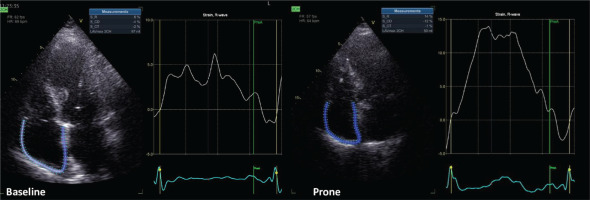
Echocardiographic images were acquired by one cardiologist (ANK) in cine-loop format from several consecutive beats and were analyzed offline (EchoPAC version 204; GE Healthcare RA) by another cardiologist (AK) with more than five years of experience in advanced critical care echocardiography. As marker of left ventricular diastolic function, left atrial strain was measured6. As conventional marker of right ventricular function, tricuspid annular plane systolic excursion (TAPSE) was measured. Right atrial longitudinal global strain was assessed using dedicated speckle-tracking software for the left atrial strain (given that software for right atrial strain is not yet commercially available). Echocardiographic assessment showed that right atrial strain (Figure 1) improved during prone ventilation and remained improved after supine repositioning.
DISCUSSION
We reported the effect of prone ventilation on right atrial strain in a patient with severe ARDS after optimization of lung recruitment and hemodynamics using esophageal pressure and pulmonary artery catheter measurements, respectively. We observed that prone ventilation was associated with an improvement in terms of lung recruitment and right atrial strain.
In this patient, prone ventilation improved pulmonary parameters. Specifically, it substantially improved oxygenation leading to a partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2:FiO2) greater than 300. This meant that the oxygenation criterion of the Berlin definition of ARDS was not met any more within 24 hours following intubation, i.e. the patient had rapidly improving ARDS. This subphenotype of rapidly improving ARDS had been recognized before the pandemic as increasingly prevalent (15–24% of all ARDS cases)7, even though it appears less prevalent during the pandemic8. Also, prone ventilation led to lung recruitment allowing for a decrease of external PEEP by 3 cmH2O while maintaining positive transpulmonary pressure at end-expiration. Measuring esophageal pressure with a balloon might have ensured optimal PEEP titration9 and, subsequently, might have allowed for reliable advanced echocardiographic measurements.
In this patient, prone ventilation was associated with an improvement in terms of right atrial strain. This improvement was observed as early as one hour after proning, was present at the end of the prone session and, importantly, sustained after supine repositioning. Right atrial strain was previously shown to be an important prognostic marker in precapillary pulmonary hypertension10,11. Interestingly, right atrial strain only modestly correlated with traditional markers of right ventricular dysfunction (namely, TAPSE and RVFWS)10 and, therefore, it was thought to have additive prognostic usefulness to RVFWS among patients with precapillary pulmonary hypertension11. Among patients with ARDS, data on right atrial strain are missing. This may be the first report of right atrial strain measurement in a patient with ARDS, who underwent prone ventilation.
CONCLUSION
Under the reported conditions, prone ventilation of our patient was associated with a sustained improvement in terms of lung recruitment and right atrial strain. Although we fully acknowledge that obesity, patient position and high PEEP made acquisition of images technically challenging and might have affected quality of strain measurements, we hope that this case might encourage clinicians to consider advanced echocardiography when managing patients under prone ventilation12. The present case also shows that combining detailed physiological and advanced echocardiographic assessment provides insights into the effect of prone ventilation in ARDS and may encourage relevant in-depth research.