INTRODUCTION
Asthma is a heterogeneous chronic inflammatory disease of the airways affecting more than 300 million people worldwide1. About 3–10% of asthmatics suffer from severe asthma (SA) that is either partially controlled or uncontrolled despite intensive treatment2,3. Severe uncontrolled asthma has been associated with impaired health-related quality of life (QoL), increased mortality and hospitalization, as well as a serious socio-economic burden due to increased healthcare resource utilization (HCRU) and derived costs2,4,5.
Based on the underlying pathophysiologic mechanisms: 1) SA is divided into two major endotype categories: T-helper (Th) 2-high (T2-high) disease, manifested by eosinophilia and pathways activated by cytokines produced by Th2 or group 2 innate lymphoid cells (ILCs); and 2) T2-low, characterized by neutrophilic or less commonly paucigranulocytic airway inflammation with normal levels of eosinophils and a lack of response to corticosteroid therapy3,6-8. T2-low is mostly classified as asthma in which the markers of T2-high disease, such as high sputum and blood eosinophil counts (BEC) and high fractional exhaled nitric oxide (FeNO), are absent3,9. However, there are no universally accepted thresholds for the aforementioned T2-high markers, the levels of which depend on various factors, such as infections, exposure to allergens, anthropometric characteristics, smoking habits, and current corticosteroid treatment3,10,11. The aforementioned lack of a clear universal definition may explain the wide variation in the reported prevalence of T2-low asthma, ranging from one-fifth to one half of individuals with asthma12-15 and from 9% to 43% among SA patients16-18.
In the absence of a consistent asthma phenotype classification system and well-defined therapeutic targets, T2-low disease represents a major unmet therapeutic challenge. Despite advances in the design of therapies for SA, most currently approved biologic agents target signaling pathways mediated by the action of the cytokines interleukin (IL)-4, IL-5 and IL-13, which play an important role in the pathogenesis of T2-high, but are less involved in T2-low disease, hence these agents are approved for eosinophilic asthma8,19-21.
Several mediators of neutrophilic and paucigranulocytic airway inflammation are being investigated as possible therapeutic targets for T2-low disease, with an increased attention to the group of cytokines on the epithelial layer of the lung referred to as alarmins, including the thymic stromal lymphopoietin (TSLP), IL-33 and IL-259,22. Alarmins instigate inflammatory responses via numerous downstream pathways, including not only T2 (IL-4, IL-13 and IL-5) but also Th1- or Th17- (IL-17) driven pathways22,23. Thus, anti-alarmin therapies may have an application in both T2-high and T2-low SA22,24. Furthermore, in patients with T2-high asthma with eosinophilic exacerbations driven by multiple, simultaneously activated T2 pathways, anti-alarmins may be more effective than agents targeting a single T2 interleukin pathway22. In patients with T2-low disease, TSLP inhibition blocks a broad range of eosinophilic and neutrophilic inflammatory pathways, playing a role in non-T2 processes and promoting airway remodeling19,22,24. In neutrophilic asthma, TSLP drives the development of neutrophil-activating Th17 lymphocytes by inducing dendritic cells, while in paucigranulocytic asthma, TSLP mediates the cross-talks between mast cells, and airway structural cells (epithelial cells, fibroblasts, and smooth muscle cells)25.
Recently, a first-in-class anti-alarmin antibody was approved in the US as add-on maintenance treatment for SA with no biomarker limitation19,22,24,26-28, while various other anti-alarmins are also in early-stage clinical development20,22, altogether presenting a promising therapeutic approach for SA patients including the distinct subpopulation of T2-low disease.
PHOLLOW was designed taking into consideration the relative paucity of information regarding the prevalence and characteristics of the SA T2-low endotype in Greece, as well as the anticipated introduction of several new treatment modalities in SA over the next years. The study aims to evaluate the prevalence and burden of T2-low disease using a new well-defined classification scheme proposed herein, as well as to characterize the patient profile, clinical features, and treatment strategies, in T2-low SA patients managed in routine care settings in Greece, thus providing input to healthcare policy-makers.
METHODS
Study population and subpopulations
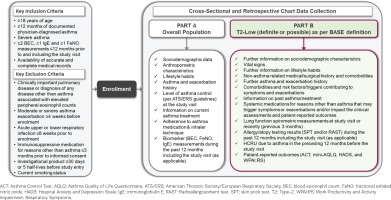
Eligible participants are male or female patients with SA as defined in Table 12,29, ≥18 years of age at the time of informed consent signature, with documented physician-diagnosed asthma for at least 12 months. During the 12 months prior to enrolment, patients need to have at least two BEC, one immunoglobulin E (IgE), and one FeNO measurements available; alternatively, such measurements need to be planned by the physician to be performed at the study visit, as per their routine practice and independently of their decision to include the patient in the current study, and thus be available at enrolment. For patients who are treated with omalizumab on the day of enrolment, at least one IgE measurement must be available before omalizumab initiation. All participants must provide written informed consent prior to inclusion in the study. Key eligibility criteria are listed in Figure 1.
Table 1
Definition of terms
| Term | Definition | |
|---|---|---|
| Allergic/atopic status | At least 2 of the following: a) total immunoglobulin E≥100 IU/mL; b) skin prick test positivity; c) earlyonset asthma (before 18 years of age). | |
| Asthma symptom control level based on ACT | Well-controlled (ACT score 20–25) | Controlled |
| Not well-controlled (ACT score 16–19) Very poorly controlled (ACT score 5–15) | Uncontrolled | |
| Clinically significant asthma exacerbation (CSE) | Worsening of asthma symptoms that requires any of the following: a) treatment with systemic corticosteroids for at least 3 days; b) an increase of the maintenance dose of oral corticosteroids for at least three days or a single depo-injectable dose of corticosteroids; c) an emergency department visit that required use of systemic corticosteroids; d) hospitalization. | |
| Current smoking status | A subject who has smoked ≥100 cigarettes in his/her lifetime, and who currently smokes cigarettes either every day or some days, or smoked in the past 30 days. | |
| Response to biological and/or mOCS treatment | Reduction of exacerbations and/or maintenance oral corticosteroid reduction by ≥50% based on the 12-month baseline period. | |
| Severe asthma (SA) | Receipt of high-dose inhaled corticosteroids/long-acting beta agonist, or medium or high dose inhaled corticosteroids/long-acting beta agonist plus long-term oral corticosteroids (with or without other add-on treatment) for at least 3 months to prevent it from becoming ‘uncontrolled’ or which remains ‘uncontrolled’ (defined below) despite this therapy (after excluding poor inhaler technique/adherence, based on physician’s judgement, comorbidities that contribute to poor asthma control and exposure to sensitizing agents/irritants)2,29. | |
| Uncontrolled asthma | At least one of the following (per ATS/ERS guidelines):
| |
[i] ACQ: Asthma Control Questionnaire. ACT: Asthma Control Test. ATS/ERS: American Thoracic Society/European Respiratory Society. GINA: Global Initiative for Asthma. NAEPP: National Asthma Education and Prevention Program. FEV1: forced-expiratory volume in 1 second. FVC: forced vital capacity. mCOS: maintenance oral corticosteroids.
Patients will be classified by SA phenotype using two definitions, based on a published consensus-driven algorithm previously used to categorize patients enrolled in the International Severe Asthma Registry30, with differentiations newly proposed herein according to expert’s input in the study team. These two definitions, namely BASE and STRICT, will classify patients into ‘T2-low (definite or possible)’, ‘definite T2-low’, and ‘possible T2-low’; and ‘T2-high (definite or possible)’, ‘definite T2-high’, and ‘possible T2-high’. Given that T2 low is characterized on the basis of non-detection of markers of T2 inflammation, and in order to be more aligned with international terminology30, ‘definite T2-low’ could also be referred to as ‘unlikely T2-high’, ‘possible T2-low’ as ‘least likely T2-high’, ‘possible T2-high’ as ‘likely T2-high’, and ‘definite T2-high’ as ‘most likely T2-high’. Both definitions will be based on the same composite measure of airway inflammation markers (i.e. BEC and FeNO), allergic/atopic status and age-of-onset phenotype (as defined in Table 1), but will employ different airway inflammation marker thresholds, also taking into consideration the current receipt of biologic and/or maintenance oral corticosteroid (mOCS) treatment and response to this treatment (as applicable) as presented in Figures 2 and 3. Patients within each T2-low subpopulation (‘definite T2-low/unlikely T2-high’, and ‘possible T2-low/least likely T2-high’) will be further stratified into subgroups based on their asthma symptom control level at the study visit using the Asthma Control TestTM (ACT) into ‘controlled’ (ACT score ≥20) and ‘uncontrolled’ (ACT score <20).
Study objectives
The primary objective of this study is to assess the prevalence of T2-low asthma phenotype using the BASE definition among patients with SA treated in routine care settings in Greece. Secondary objectives include assessment of the prevalence of T2-low asthma phenotype using the STRICT definition, assessment of the level of asthma symptom control in the T2-low subpopulation [‘overall (definite or possible)’, ‘definite’, and ‘possible’, as per BASE and STRICT definitions separately], as well as characterization of the patient demographic and clinical profile, current and past therapeutic management strategies, clinically significant asthma exacerbation (CSE) burden (as defined in Table 1) over the past 12 months, and patients’ lung function, as assessed by spirometry at the study visit, in the T2-low subpopulation (using the BASE and STRICT definitions separately) and its subgroups by asthma symptom control level (‘controlled’ and ‘uncontrolled’). Furthermore, the study will assess the asthma-specific QoL using the Mini – Asthma Quality of Life Questionnaire (Mini-AQLQ), the anxiety and depression levels using the hospital anxiety and depression scale (HADS), the work productivity loss and activity impairment using the Work Productivity and Activity Impairment: Respiratory Symptoms (WPAI:RS) questionnaire at the study visit, the asthma-related HCRU in the past 12 months before the study visit, and the frequency of OCS-dependent patients, in the T2-low SA subpopulation and its subgroups by asthma symptom control level.
Exploratory objectives of this study include the assessment of the influence of selected patient and disease characteristics on the prevalence of T2-low SA (according to the BASE definition); the identification of potential patient, treatment and disease characteristics influencing ACT-assessed asthma symptom control in the T2-low SA subpopulations as per the BASE and STRICT definitions separately; and the examination of the differences in patient, disease, and treatment characteristics between the subgroups of patients with definite and possible T2-low SA in the T2-low SA subpopulations as per the BASE and STRICT definitions separately. The study objectives and the (sub)populations to which they refer are summarized in Table 2.
Table 2
PHOLLOW study objectives in the populations of interest
| No. | Objectives | Overall SA | T2-Low Subpopulation | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Defined using BASE | Defined using STRICT | |||||||||||||||||||
| Overall | Definitea | Possibleb | Overall | Definitea | Possibleb | |||||||||||||||
| (U) | C | U | (U) | C | U | (U) | C | U | (U) | C | U | (U) | C | U | (U) | C | U | |||
| Primary objective | ||||||||||||||||||||
| 1 | Prevalence of T2-low SA based on the BASE definition | ✓ | ||||||||||||||||||
| Secondary objectives | ||||||||||||||||||||
| 1 | Prevalence of T2-low SA based on the STRICT definition | ✓ | ||||||||||||||||||
| 2 | Level of asthma symptom control using the ACT | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| 3 | Characterization of patient demographic and clinical profile | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 4 | Current and past therapeutic management strategies | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 5 | Asthma exacerbation burden over the past 12 months | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 6 | Patient’s lung function (assessed by spirometry) at the study visit | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 7 | Asthma-specific QoL at the study visit | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 8 | Anxiety and depression levels at the study visit | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 9 | Work productivity and activity impairment at the study visit | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 10 | Asthma-related HCRU in the 12 months before study visit | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 11 | Frequency of OCS-dependent patients | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Exploratory objectives | ||||||||||||||||||||
| 1 | Influence of patient/disease characteristics on the prevalence of T2-low SA based on the BASE definition | ✓ | ||||||||||||||||||
| 2 | Factors influencing asthma symptom control | ✓ | ✓ | |||||||||||||||||
| 3 | Different characteristics between definite/possible T2-low SA | ✓ | ✓ | |||||||||||||||||
Study design
PHOLLOW (a real-world, multicenter, cross-sectional and retrospective chart review study to assess the prevalence of asthma inflammatory PHenotypes and to characterize the patient prOfiLe, clinical features and disease burden of type-2 LOW severe asthma in routine care settings in Greece) is an epidemiological, single-country, multicenter, cross-sectional with retrospective chart review two-part study, with a single-visit data collection schedule. The study will include a representative sample of outpatients with SA treated by asthma specialists (pulmonologists and allergists) in routine care settings in Greece. The planned recruitment period, and, consequently, the expected duration of the study is 12 months, but will actually be determined by achievement of the target sample size. Each patient’s look-back period for data collection from medical charts extends from the date of asthma symptom onset to the date of obtainment of informed consent. The study design including data collection variables at the study visit is summarized in Figure 1.
In part A of the study, appropriate top-level patient data are collected from all enrolled eligible SA patients to assess the prevalence and identify potential predictors of T2-low SA. The most recent BEC/FeNO/IgE measurements recorded in the 12-month period prior to and including the study visit, along with allergic/atopic status, current receipt of biologic and/or mOCS treatment, and response to biologic and/or mOCS treatment are used for stratification of patients into SA phenotypes; an exception applies for patients treated with omalizumab at the study visit, for whom the most recent IgE measurement before omalizumab initiation is used for determining the allergic/atopic status, in case that the patient meets one of the other two qualifying conditions [i.e. Skin Prick Test (SPT) positivity or Early-Onset Asthma (EOA)]. SPT results are collected, if needed, prior to omalizumab initiation. For patients classified as T2-high SA according to the BASE definition, study participation ends in part A, and no further data are collected about them.
Part B of the study only concerns patients classified as T2-low as per the BASE definition and involves further data collection and patient-reported outcome (PRO) assessments, in order to address the applicable study secondary, and exploratory objectives.
The study is designed and will be reported in accordance with the ethical principles laid down in the Declaration of Helsinki, the Guidelines for Good Pharmacoepidemiology Practice of the International Society for Pharmacoepidemiology, the STROBE guidelines where applicable, the EU General Data Protection Regulation, and the local rules and regulations.
Site selection and patient enrolment
Candidate investigators originate from a pool of specialists, both pulmonologists and allergists, across Greece, with efforts made to recruit sites with representative medical practices and to provide data describing the many real-life aspects of asthma management and its burden on patients and the healthcare system.
Site selection has been performed in a non-random manner, through a documented and constructed feasibility assessment process accounting, among other factors, for physicians’ qualifications, number of potentially eligible patients, and representativeness based on hospital’s patient care activity (number of cared SA patients during the 12-month period before start of recruitment), hospital location (geographical region), and type of healthcare site/institution (publicly owned non-academic site/institution, university hospital, privately-owned hospital, private practice). Based on preliminary feasibility projections, between 20 and 40 patients are to be recruited by each site, with the planned number of sites being between 20 and 30.
To minimize patient selection bias, physicians are requested to consecutively enroll the first eligible patients attending their clinic/office over the pre-specified study recruitment period. To enable assessment of potential selective enrolment, physicians are asked to complete a screening log of all potentially eligible patients, i.e. of patients who meet study eligibility criteria regardless of whether they agree or are able to participate or not, including the minimum required information of date seen, sex, age range on the date seen, and reason for non-consenting.
Data collection
The study involves both primary and secondary data collected by means of a web-based data capture system [electronic Case Report Form (eCRF)] adhering to all applicable data protection regulations and requirements with regard to electronic records and database validation.
Data are collected directly by the participating physicians as generated within the normal clinical practice setting, through routine clinical assessments performed in the context of the single study visit as well as patient self-report (where applicable) and use of PROs. Data collection includes the variables and measurements that are described in Figure 1 and Table 3. Patient source data pertaining to medical and asthma-related history, exacerbation history, hospital-based HCRU over the past 12 months, results of airway inflammation biomarkers of interest, and asthma management practices are abstracted from patients’ medicals records and patient self-report. PROs are completed by the patients themselves via self-administered paper questionnaires of the validated Greek versions (Table 4)31-34. The scores/answers are subsequently transcribed into the eCRF directly by the participating physicians.
Table 3
Study outcome measures corresponding to study objectives
| Objective | Outcome measure |
|---|---|
| Primary outcome | |
| 1 | Proportion of patients with T2-low SA (BASE definition) |
| Secondary outcomes | |
| 1 | Proportion of patients with T2-low SA (STRICT definition) |
| 2 | Proportions of patients with ‘well-controlled’, ‘not well-controlled’ and ‘very poorly controlled’ asthma symptoms (as defined in Table 1) |
| 3 | Patient’s demographic and clinical characteristics |
| 4 | Types and frequencies of current non-pharmacological and pharmacological treatments by drug class, and drug utilization patterns (including dose, dosing frequency, route of administration, and duration of treatment) Types and frequencies of past pharmacological treatment modalities by drug class |
| 5 | CSE rate (expressed as the number of CSEs per patient) over the past 12 months |
| 6 | Pre-bronchodilator FEV1, FVC, FEV1/FVC, and FEF25-75% (absolute and percent predicted values, as applicable) at the study visit |
| 7 | Mini-AQLQ total and domain scores at the study visit Proportion of patients with impaired QoL due to asthma (i.e. with Mini-AQLQ total score <6) at the study visit |
| 8 | HADS total and subscale (HADS-A and HADS-D) scores at the study visit Proportion of patients with symptoms of anxiety and/or depression as indicated by HADS-A and/or HADS-D score ≥11 at the study visit |
| 9 | WPAI:RS domain scores referring to absenteeism, presenteeism, and work productivity loss,among patients who are employed, at the study visit WPAI:RS domain score referring to activity impairment among all patients, at the study visit |
| 10 | Number of hospital admissions with a primary discharge diagnosis of asthma (ICD-9-CM 493), of unscheduled visits to private practices, and of ED visits due to asthma in the past 12 months before the study visit Annual hospital admission and ED visit rates Inpatient LOS (overall LOS, ICU/HDU LOS, and non-ICU/HDU LOS) in the past 12 months before the study visit |
| 11 | Proportion of patients treated with OCS equivalent to a daily dose of ≥5 mg prednisone for ≥3 continuous months directly preceding the study visit |
| Exploratory outcomes | |
| 1 | Association of selected patient and disease characteristics with the prevalence of T2-low SA (BASE definition) |
| 2 | Association of treatment, demographic and clinical characteristics with asthma symptom control (continuous/ categorical ACT) |
| 3 | Patient, disease, and treatment characteristics differing between the subgroups of definite and possible T2-low SA patients |
[i] ACT: Asthma Control Test. AQLQ: Asthma Quality of Life Questionnaire. ED: Emergency Department. FEF: forced expiratory flow. FEV1: forced-expiratory volume in 1 second. FVC: forced vital capacity. HADS: Hospital Anxiety and Depression Scale. HADS-A: HADS-Anxiety subscale. HADS-D: HADS-Depression subscale. HDU: Hight Dependency Unit. ICD-9-CM: International Classification of Diseases-9-Clinical Modification. ICU: Intensive Care Unit. LOS: length of stay. OCS: oral corticosteroids. QoL: quality of life. SA: severe asthma. T2: Type-2. WPAI:RS: Work Productivity and Activity Impairment: Respiratory Symptoms.
Table 4
Patient-reported outcome instruments used in the study
| Instrument | Main outcomes/ domains/ subscales | Number of items | Recall period | Scale/score | Average time to complete |
|---|---|---|---|---|---|
| Mini-AQLQ31 | Symptoms, activity limitation, emotional function, environmental stimuli | 15 | 2 weeks | 7 points each question | 3 –5 min |
| Asthma Control Test32 | Frequency of shortness of breath and general asthma symptoms, use of rescue medications, effect of asthma on daily functioning, overall self-assessment of asthma control | 5 | 4 weeks | 5 points each item | 30 sec |
| Hospital Anxiety and Depression Scale33 | Anxiety, depression | 14 | 1 week | 4 points each item | 2 –5 min |
| Work Productivity and Activity Impairment: Respiratory Symptoms34 | Absenteeism, presenteeism, and daily activity impairment | 6 | 1 week | 0–100% each outcome | 2 –3 min |
In line with the purely observational and non-interventional nature of the study, no further clinical, laboratory, imaging, and lung function assessments are required apart from those performed as per the treating physician’s routine medical practice.
Internal validity of the outcomes is safeguarded to the extent feasible with the implementation of appropriate source data verification and quality assurance measures.
Sample size
This study’s primary aim is purely descriptive in nature. A sample size of 600 patients, offers a margin of error of 4.1% [with 95% confidence interval (CI) using the Clopper-Pearson exact method ranging between 45.9% and 54.1%] for the estimation of a percentage of 50% where the width of the CI is largest. Assuming that a proportion of at least 20% in the overall eligible population will be classified as T2-low according to the BASE definition, the study size that will be used for analysis of the applicable secondary objectives is expected to exceed 100 patients. This means that regarding any qualitative variable observed at any frequency >0%, the margin of error would range from >3.6% to 10.2%.
Statistical analysis
All analyses will be performed in the set of eligible enrolled patients among each subpopulation of interest provided that the number of patients included in each subgroup is sufficient and allows for meaningful estimates.
Continuous variables will be expressed using descriptive statistical measures, while categorical variables will be expressed using frequency tables. The 95% Clopper-Pearson CI for binomial proportions and the 95% Poisson CI for incidence rates will be estimated. The normality of distribution of continuous variables will be examined using the Shapiro-Wilk test. Comparisons of continuous variables will be performed using the t-test (parametric) or Mann-Whitney U (non-parametric) test for two independent samples, while comparisons of categorical variables will be performed using either the Pearson’s chi-squared test or Fisher’s exact test.
The association of patient, treatment and disease characteristics with binary and continuous outcomes will be evaluated through univariable and multivariable binary logistic regression and linear regression models, respectively. In regard to multivariable regression analysis, the stepwise procedure based on the minimization of the Akaike’s information criterion will be applied for the final model selection. All models will be fitted, provided that the number of patients included is sufficient to allow for meaningful inferences. In addition, the type of institution (academic/non-academic) and the administrative geographical region of the study site (Attica, outside Attica) will be considered as potential confounders on the association of patient and disease characteristics with the prevalence of T2-low SA and asthma symptom control level.
No imputation methods will be applied for missing data, with the exception of partial dates. With regard to the scoring and handling rules of any missing PRO items, the relevant scoring instructions, where available, will be followed. All statistical tests will be two-sided and will be performed at a 0.05 significance level.
DISCUSSION
PHOLLOW aims to provide epidemiological data on asthma phenotypes of SA patients treated in routine care in Greece, and to characterize the patient profile, clinical features, and disease burden of T2-low SA. The stratification of patients in SA phenotypes will be based on a clearly defined scoring system and criteria utilizing BEC, FeNO, allergic/atopic status, current receipt of biologics and mOCS treatment, as well as response to such treatment. The study will employ two different definitions, termed BASE and STRICT, which use the same criteria and scoring, yet differ in the classification of patients into T2-low or high phenotypes by cumulative score. In particular, for patients not receiving biologic or mOCS treatment, STRICT uses a more stringent BEC threshold (150 versus 300 cells/μL). Consequently, among this treatment subgroup, a proportion of patients categorized as ‘definite T2-low/unlikely T2-high’, ‘possible T2-low/least likely T2-high’, and ‘possible/likely T2-high’ by BASE will be re-classified as ‘possible T2-low/least likely T2-high’, ‘possible/likely T2-high’, and ‘definite/most likely T2 high’ by STRICT, respectively, similar to another recently published approach based on an expert consensus framework categorizing patients from the International Severe Asthma Registry30. Among biologic and/or mOCS treated patients, those classified as ‘definite T2-low/unlikely T2-high’ and those classified as ‘possible T2-low/least likely T2-high’ by the BASE will be re-classified as ‘possible T2-low/least likely T2-high’ and ‘possible/likely T2-high’ by the STRICT definition, respectively. Thus, the STRICT definition will result in a smaller prevalence of T2-low among the overall SA study patient population.
The algorithm that will be used for the classification of patients into SA phenotypes is newly proposed in the present study and aims to fill a gap, particularly in the definition of T2-low. Earlier approaches to characterize T2-low asthma defined this phenotype based on the mere absence of T2-high markers. Over the past years, several studies have adopted more specific criteria for this definition, using selected biomarkers and threshold values. For instance, Jackson et al.18 characterized patients as T2-low if BEC was <300 cells/μL and FeNO was <25 ppb; a publication17 of the UK Severe Asthma Registry defined T2-low as BEC <150 cells/μL and FeNO<25 ppb, another publication16 of the Belgian Severe Asthma Registry defined T2-low as induced sputum eosinophilic count <3% or FENO and BEC <27 ppb and <188/mm3, respectively, while the publication of the data from the International Severe Asthma Registry classified eosinophilic and non-eosinophilic phenotypes based on a combination of clinical and biomarker variables including the highest BEC ever (≥300, ≥150–300, or <150 cells/mL), anti-IL-5/5 receptor treatment, long-term OCS use ever, elevated FENO (≥25 ppb) ever, nasal polyps diagnosis ever, and adult asthma onset (≥18 years)30. Recently, a composite biomarker scoring system using three biomarkers, each assigned a score of 0, 1, or 2, was tested as a strategy to adjust corticosteroid dose in patients with SA. The algorithm used FENO (<15, 15–29, ≥30 ppb), BEC (<150, 150–299, ≥300 cells/μL), and serum periostin (<45, 45–54, ≥55 ng/mL), and was compared with a standardized symptom-risk based algorithm, without significant differences found in the proportion of patients with reduced corticosteroid dose between the two strategies35.
PHOLLOW builds upon prior knowledge, but also expands it with incorporation of additional parameters to construct a novel SA phenotype classification system. In particular, biomarkers like BEC and FeNO, which are among the most widely used criteria for patient stratification into SA phenotypes, are maintained in the PHOLLOW algorithm. On the other hand, along with the aforementioned more traditional biomarkers, the new algorithm also takes into account allergic/atopic status (total IgE levels, age of asthma onset, and SPT positivity), current treatment with biologics and/or mOCS, and response to such treatment (as applicable). It should be noted that the use of biologics and/or mOCS is a very critical parameter to consider when assessing patients’ asthma phenotype, as it can downregulate T2 biomarkers and lead to misclassification11. Regarding the weight of each component in the cumulative score, a 3-point (0, 1, or 2) scoring system is applied for continuous variables (i.e. BEC, FENO), and a 2-point (0 and 1, or 0 and 2) score for dichotomous (answered by yes or no) variables (allergic/atopic status, response to therapy). The specific cut-off values selected for the continuous variables herein have been previously used in studies of SA therapeutics, and have even shown predictive value in terms of therapeutic benefit in some cases36. Response to biologic/mOCS treatment is scored with the maximum possible component score (2) in all cases, as it is considered an indication of T2 inflammatory mechanisms in action35. Individual component scores are combined in a composite scoring system, selected as it is thought to provide a systematic yet simple method of classification, which would be more reliable than any single item in the measure. Lower cumulative scores favor towards stratification to T2-low, while higher scores will tend to classify patients as T2-high. T2-low and T2-high phenotypes are further subdivided into ‘definite’ and ‘possible’. The resulting stratification can be viewed as a gradient, with ‘definite T2-low/unlikely T2-high’ and ‘definite/most likely T2-high’ positioned in the two extremes, which flank the intermediate ‘possible T2-low’ and ‘possible T2-high’ phenotypes. The use of two different cut-offs for BEC for classification scoring, namely 150 and 300 cells/μL in the STRICT and BASE definition, respectively, has been adopted to minimize information bias with respect to the primary outcome of the T2-low population prevalence, in the absence of a universally accepted classification scheme. The results generated herein are expected to be meaningful in the broader context of disease pathobiology and emerging therapies.
Limitations
There are several limitations inherent to the observational cross-sectional and retrospective design. Mechanisms have been put in place to mitigate confounding due to potential patient selection bias, as well as information/recall and response bias in patient report through use of a consecutive sampling process, validated and standardized instruments involving short recall periods, and specific timing for PRO completion before study-related assessments, respectively. To account for potential confounding on the primary study outcome, the possible influence of confounding factors will be examined through multivariable analyses, as an exploratory outcome. It should also be noted that although efforts have been made to recruit sites with representative medical practices, the sampling frame was based on a non-probability technique, with physicians being selected in a non-random manner; thus, the generalizability of the study outcomes is indeterminate. Another foreseen limitation of PHOLLOW pertains to the sample size that will be eventually used for assessment of the secondary and exploratory objectives, since the actual prevalence of T2-low is unknown. Nevertheless, the number of T2-low patients in the study population is expected to exceed 100, which is considered sufficient to characterize with acceptable precision this subpopulation. Systematic error arising from inaccurate measurements or missing data is expected to be mitigated by having as a prerequisite for study eligibility the availability of sufficient relevant medical records. Nonetheless, inaccuracies in the assessment of asthma-related HCRU in the last 12 months before enrollment may still be present, as visits performed in an outpatient setting may not be fully recorded in the patients’ records.
Despite these limitations, studies of such design essentially, through collecting data about real-world management practices and their outcomes, help to bridge clinical research in strictly controlled randomized settings with daily clinical practice. As real-world studies follow less restrictive methodological standards than controlled trials in terms of patient selection, treatment, and other design aspects, their results are better generalizable, especially for populations with diseases of complex and heterogeneous biology, such as asthma. Moreover, enrolment of patients from diverse locations throughout Greece and different types of healthcare institutions, will further enhance the generalizability of the findings.
CONCLUSIONS
Evidence generated through the PHOLLOW real-world, multicenter, cross-sectional and retrospective chart review study is anticipated to provide valuable input into the local treatment landscape and unmet needs in the specific setting of T2-low SA in clinical practice, which is generally lacking. In light of the expected position of novel treatments in the SA pathway, the outcomes of the present study will facilitate the future evaluation of generalizability and relevance of clinical trial data to the real-world in Greece, which is increasingly becoming essential in clinical decision making.