INTRODUCTION
Asthma is a chronic respiratory disease that encompasses a broad spectrum of phenotypes and endotypes1. Approximately 5–10% of patients with asthma suffer from severe disease, which cannot be adequately controlled by conventional treatment with a high dose of inhaled corticosteroids combined with long-acting β2-agonists or oral corticosteroids (OCS), or control is lost when deescalating treatment. Based on a 2021 report of the International Severe Asthma Registry, in >80% of patients with severe asthma, the disease is characterized by the presence of eosinophilia, which causes inflammation and hyperresponsiveness of the airways, and is referred to as severe eosinophilic asthma (SEA)2,3.
Patients with SEA experience substantial symptom burden and frequent disease exacerbations, which contribute to disease-related morbidity and progressive loss of lung function. Persistent symptoms and frequent exacerbations also conduce toward patients’ activity limitation, which, along with compromised sleep quality and feelings of anxiety or depression, adversely and substantially influence their health-related quality of life (HRQoL), leading to a considerable psychosocial and economic disease burden. Among asthmatic patients, both anxiety and depression have been associated with worse perception of asthma control4, while poorly controlled disease has been associated with greater healthcare resource utilization (HCRU), elevated direct and indirect healthcare costs, as well as higher scores for work presenteeism and absenteeism5.
Among the currently available monoclonal antibodies (mAb) for add-on biologic treatment of SEA, benralizumab was approved in 2017 and 2018 in the USA and Europe, respectively. Benralizumab is a humanized afucosylated immunoglobulin G1 kappa mAb that binds to the alpha subunit of the interleukin (IL)-5 receptor on the surface of eosinophils and basophils, inhibits IL-5-mediated proliferation and potently induces antibody-dependent cell-mediated cytotoxicity to these cells6-9. Through this mechanism, benralizumab mediates reduction of eosinophilic inflammation. In the placebo-controlled pivotal trials SIROCCO7 and CALIMA8, benralizumab significantly reduced the annual exacerbation rate (AER) and improved asthma symptoms and lung function. Patients with higher baseline blood eosinophil counts (BEC) experienced an improved treatment response compared to those with lower baseline BEC, particularly regarding forced-expiratory volume in 1 second (FEV1)7,8. The derived benefit of benralizumab was also extended in adults with severe uncontrolled asthma with BEC ≥150 cells/μL in terms of reducing the AER, and improving FEV1 and total asthma symptom scores10, as well as in patients with fixed airway obstruction in terms of reducing the AER and improving symptoms and lung function11. Furthermore, in the phase III trial ZONDA, benralizumab significantly lowered the need for OCS while asthma control was maintained in patients with severe asthma and increased eosinophil count9. The extension trial BORA12 and two 2-year integrated analyses have confirmed the long-term efficacy and safety of benralizumab, as well a sustained OCS sparing effect in patients with uncontrolled SEA13. In addition to the aforementioned clinical outcomes, evidence generated in clinical studies also supports that patients treated with benralizumab also experienced improvement in their HRQoL and overall health status as assessed using the Asthma Quality of Life Questionnaire, the St. George’s Respiratory Questionnaire (SGRQ), as well as the Clinician and the Patient Global Impression of Change scores (CGIC and PGIC, respectively)7,8,14-16.
To complement and expand the data generated in the pre-authorization clinical trial setting in a broader patient population, but also to gain insight into the patient-perceived treatment benefit, real-world studies have been conducted17-21. However, given the relatively recent emergence of benralizumab in the market, such evidence remains limited overall and is non-existent in Greece. EMPOWAIR was designed to generate real-world evidence (RWE) on a broad range of well-established clinical and novel patient-centered outcomes, which are critical for the assessment of the therapeutic benefit of benralizumab in SEA both from the physician’s and the patient’s perspective. The study will employ a multi-aspect and patient-centric approach, and will engage advanced remote patient monitoring technologies using the Air Next portable spirometer (NuvoAir AB, Stockholm, Sweden) and the Fitbit® wristband wearable activity tracker (WAT), to collect data on patients’ lung function parameters and physical activity. All main study outcomes will be examined at various timepoints throughout the observation period, starting as early as 4 weeks after treatment initiation, thus enabling the identification of ‘early’ treatment responders with a closer focus on patients’ physical and psychological well-being and HRQoL in addition to asthma control, achievement of remission, and lung function metrics.
METHODS
Study design, setting, and population
EMPOWAIR (‘A rEal-world, Multicenter, 48-week Prospective cohort study to capture clinical and patient-centered Outcomes in adults With severe eosinophilic Asthma treated wIth benRalizumab in routine care settings in Greece’) is a single-country, non-interventional, multicenter, 48-week prospective cohort study, mainly based on primary data collection, which will include adult patients with SEA initiated on benralizumab in routine care settings of Greece. The study will be carried out by 20–25 sites comprising private practices and hospital clinics specializing in the management of asthma in geographically diverse locations throughout Greece, with a balanced representation of public, academic, and private sectors. All aspects of treatment and clinical management of patients will be in accordance with local clinical practice and applicable local regulations, and at the discretion of the participating physicians, with no changes to the current standard of care required for study participation. The study will enroll patients who have been prescribed benralizumab (Fasenra®) prior to informed consent obtainment and independently of study participation and will be treated according to the local prescribing information (Summary of Product Characteristics)22 of the study medication and routine medical practice in terms of visit frequency and type of assessments performed.
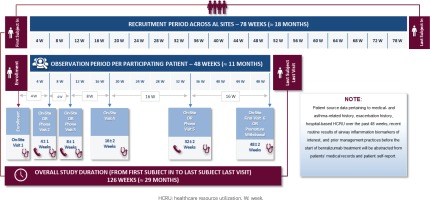
The overall duration of the study is expected to be approximately 126 weeks (about 29 months), including a recruitment period of 78 weeks and an observation period of 48 weeks. Follow-up visit frequency will be determined by the treating physician; however, study-related data will be collected at study enrollment and at 4, 8, 16, 32, and 48 weeks post-index (i.e. after benralizumab treatment initiation) with an allowable time window of ±1 to ±2 weeks, depending on the specific data collection timepoint. Data collection at enrollment and at 16 and 48 weeks post-index will be performed in the context of on-site routine visits at the private practices/hospital clinics, whereas data collection at 4, 8, and 32 weeks post-index will take place through either on-site routine visits or telephone contacts at physicians’ discretion, based on which scheduling option better accommodates patients’ preferences and needs for care. An overview of the study design is presented in Figure 1.
The eligibility criteria for participation in the study are listed in Table 1, while relevant definitions are provided in Table 2.
Table 2
Definition of terms
[i] ACQ-6: asthma control questionnaire-6. ACT: asthma control test. FEV1: forced-expiratory volume in 1 second. FVC: forced vital capacity. MCID: minimum clinically important difference. NAEPP: national asthma education and prevention program. OCS: oral corticosteroids. SGRQ: St. George’s respiratory questionnaire.
Research objectives
The study primarily aims to assess the change from baseline in HRQoL, as measured by the SGRQ, and to estimate the proportion of patients achieving a minimum clinically important improvement in respiratory health status, defined as ≥ 4-point reduction from baseline in SGRQ total score, after 16 weeks of treatment with benralizumab. The study also has a number of secondary and exploratory objectives, which are listed in Table 3.
Table 3
Study objectives
[i] ACQ-6: asthma control questionnaire-6. CGIC: clinician global impression of change. CRSwNP: chronic rhinosinusitis with nasal polyps. CSE: clinically significant exacerbation. FeNO: fractional exhaled nitric oxide. FEV1: forced-expiratory volume in 1 second. HADS: hospital anxiety and depression scale. HCRU: health care resource utilization. HRQoL: health-related quality of life. IgE: immunoglobulin E. OCS: oral corticosteroids. PGIC: patient global impression of change. PSQI: Pittsburgh sleep quality index. SGRQ: St. George’s respiratory questionnaire. SNOT-22: sino-nasal outcome test-22. WAT: wearable activity tracker. WPAI:RS: work productivity and activity impairment: respiratory symptoms.
Site selection
Study investigators will be selected from a pool of lung specialists across Greece in a non-random manner through a documented and constructed feasibility assessment process that will account, among others, for physicians’ qualifications, previous participation and experience in similar clinical studies, recruitment potential and retention capability, geographical representativeness based on proportionality to the total Greek population, and representativeness in terms of asthma cases encountered in routine practice settings, hospital clinic/private practice location (i.e. regional setting), and type of healthcare site.
Data collection and sources
Data collection will be carried out by means of a specifically designed web-based data capture system [electronic Case Report Form (eCRF)], which will adhere to all applicable data protection regulations and requirements. The study will mainly employ primary data collected at enrollment and prospectively during the study visits or telephone contacts. All visits and examinations will be performed according to institutional standards of care and physicians’ clinical judgment. Physical activity and at-home spirometry device-derived data will be collected and accessed via a cloud-based server. Secondary data pertaining to medical- and asthma-related history, exacerbation history, hospital-based HCRU over the past 48 weeks, recent routine results of airway inflammation biomarkers of interest, and prior management practices before the start of benralizumab treatment, will be abstracted from patients’ medical records and through patient self-report. Furthermore, a patient diary will be used to record information on HCRU, rescue medication use, asthma-related nocturnal awakenings requiring rescue medication during the study observation period, and any non-asthma-related reasons that may have limited patients’ typical physical activity level during the device-wearing periods.
Study-specific physical activity data, namely step counts and active minutes, will be collected using a WAT device (Fitbit® wristband). A unique number tracker, as well as training on its use, will be provided to each participant at enrollment. Patients will be asked to wear their WAT continuously over the waking hours, excluding bathing time and device’s charging time, for at least 7 consecutive days before the first benralizumab dose and during the last week of each month post-index. Eight to ten days before each data collection time point (study visit or telephone contact, as applicable), patients will be contacted by phone by the study investigator or staff, reminding them to wear their activity tracker for the next 7 days until the study visit/telephone contact. Activity data for prespecified time points will be analyzed provided at least 4 valid days are available within that week, with valid days defined as those with a minimum of 10 hours of waking wear time.
For the home-based spirometric measurements, patients will be provided the portable hand-held Air Next spirometer at enrollment and receive comprehensive training on its use. Patients will be asked to perform home-based spirometry at least once weekly (in the morning within the first 3 hours after awakening and before the administration of any asthma-related medication) after the start of benralizumab treatment until the end of the study observation period. In addition, they will be requested to perform spirometry upon symptom worsening on a daily basis for at least 7 consecutive days. The analysis will include only valid tests (sessions), defined as those fulfilling the maneuver acceptability criteria as well as the repeatability criteria for both Forced Vital Capacity and FEV1, and being of ‘excellent’, ‘very good’, or ‘good’ quality grade based on the grading system for assessment of spirometry quality that is jointly recommended by the American Thoracic Society and the European Respiratory Society23,24. For each day, the patient’s highest spirometric values from the valid session with the best quality grade will be selected for analysis purposes.
The clinician’s perception of change in the patient’s overall health since treatment initiation will be assessed using the Greek version of the CGIC which is a 7-point Likert-type scale. In addition, the study will use a number of patient-reported outcomes (PROs), shown in Table 4.
Table 4
Patient-reported outcome instruments used in the study
| Instrument | Main outcomes/ domains/ subscales | Number of items | Recall period | Scale/score | Comments |
|---|---|---|---|---|---|
| St. George’s Respiratory Questionnaire30 | Part 1: severity of respiratory symptoms. Part 2: daily activity and psychosocial impact of respiratory condition. Yields a total score and 3 component scores (symptoms, activity, and impacts) | 50 | 4 weeks | 0 (best possible status) – 100 (worst possible status) for component and total scores | 4 unit-decrease: minimum clinically important difference. 8 unit-decrease: moderately efficacious change. 12 unit-decrease: very efficacious treatment |
| Asthma Control Questionnaire-631 | Night-time waking, symptoms on waking, activity limitation, shortness of breath, wheezing, and use of short-acting β2 agonist | 6 | 1 week | 0 (well controlled) – 6 (extremely poorly controlled) | Score change of at least 0.5 is meaningful and used to support responder definition |
| Hospital Anxiety and Depression Scale32 | Two 7-item subscales: anxiety and depression | 14 | 1 week | 0–21 for each subscale, higher scores represent worse mental health | Scores 0–7: normal. 8–10: possible cases of anxiety/depression. ≥11: likely cases of anxiety/depression |
| Pittsburgh Sleep Quality Index33 | Subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction | 19 | 1 month | 0–3 for each component, 0–21 for total score, lower scores indicate better sleep quality | A global score of ≥5 indicates poor sleep quality |
| Work Productivity and Activity Impairment: Respiratory Symptoms34 | Absenteeism, presenteeism, and daily activity impairment | 6 | 1 week | 0–100% each outcome | Scores indicate percent: a) work time missed, b) impairment while working, c) overall work impairment, d) activity impairment, due to respiratory symptoms |
| Patient Global Impression of Change35 | Perception of change in overall health | 1 | - | 1 (much better) – 7 (much worse) | Response is indicated by a score of ‘much better’ or ‘moderately better’ |
| Sino-Nasal Outcome Test-2236 | Physical problems, functional limitations, and emotional consequences of sino-nasal conditions | 2 weeks | 0 (no problem) – 5 (problem as bad as it can be) for each item, 0–110 for total score, higher scores indicate poorer outcomes | A minimal importance difference of 8.90 has been established for individual score change |
Ethics
The conduct of this study will adhere to the applicable national regulatory requirements governing the conduct of such type of clinical research. The study has been designed and will be conducted and reported in accordance with the ethical principles laid down in the Declaration of Helsinki, the Guidelines for Good Pharmacoepidemiology Practice of the International Society for Pharmacoepidemiology, the Strengthening the Reporting of Observational Studies in Epidemiology guidelines where applicable, the EU General Data Protection Regulation, and the local rules and regulations. All participants will provide signed informed consent.
Sample size
Sample size estimation has been based on the primary endpoint pertaining to the mean difference in SGRQ total score from baseline at 16 weeks after benralizumab initiation. A sample size of 128 patients is required in order to have 80% power to detect differences in means of 4 points [representing the minimum clinically important difference (MCID) for SGRQ total score] using paired t-test with a 0.05 two-sided significance level and assuming a standard deviation (SD) of the differences of 16.0 (effect size: 0.25). The assumption was based on data from the SOLANA trial, where the SD of this difference at 4, 8 and 12 weeks for the benralizumab and placebo groups ranged from 14 to 2118. This sample size offers a precision of 9.0% [with 95% confidence interval (CI) using the Clopper-Pearson exact method ranging between 41.0% and 59.0%] for the estimation of a percentage of 50% of patients achieving the MCID at the post-index time point where the width of the CI is the largest. To control for an approximate 15% non-evaluable/drop-out rate, 150 patients are finally required to ensure the aforementioned sample size for the final statistical analysis.
The sample size based on the mean difference in SGRQ total score was estimated using the nQuery 8.0 (‘Paired t-test for difference in means’), whereas the size determination based on Clopper-Pearson exact method has been performed using the statistical software package SAS v9.4 (SAS Institute, Cary, NC).
Statistical analysis
All statistical analyses and generation of tables and patient data listings will be performed using the latest version of SAS® software available at statistical analysis initiation. Continuous variables will be summarized using descriptive statistical measures, and categorical variables will be displayed using frequency tables. The normality of distribution of continuous variables will be examined using the Shapiro-Wilk test. Concerning binomial proportions, Clopper-Pearson 95% CIs will be estimated. Summary statistics for the differences between values of related continuous variables will be presented along with 95% CI for the mean difference in the context of the primary endpoint. Differences from baseline values will be assessed using the paired t-test or Wilcoxon signed-rank test for paired continuous data and the McNemar’s test for paired nominal data. The association of baseline and disease characteristics with binary or count outcomes will be evaluated through univariable and multivariable binary logistic regression and Poisson regression models, respectively. Poisson regression models will also be fitted in order to estimate the HCRU and CSE rate during the 48-week period pre- and post-benralizumab initiation, as well as the difference in the rates between these periods, along with the relevant 95% CIs. In addition, linear mixed-effects models for repeated-measures will be applied in order to estimate the mean weekly change in home-measured spirometric lung function indices over the study observation period. Time to benralizumab treatment discontinuation will be estimated using the Kaplan-Meier method. All analyses will be performed in the set of eligible enrolled patients among each population of interest specified in the study objectives; all statistical tests will be two-sided and performed at a 0.05 significance level.
DISCUSSION
EMPOWAIR employs a multifaceted approach to assess the effect of benralizumab treatment on clinical and patient-centered outcomes in patients with SEA treated in routine care settings in Greece. To this end, a number of complementary data will be collected, derived from various sources, and based on objective, physician-assessed, patient-perceived, and handheld device-derived measures. In addition to data generated through routine clinical examination, a major part of the study, including the primary endpoint, will be based on various PROs addressing asthma-related symptoms, as well as the effect of asthma on overall health status, emotional state and mental wellbeing, sleep quality, daily activities, and work productivity. Moreover, the study will make use of portable spirometers and WATs, handled by the patients themselves, to measure and record spirometric parameters and physical activity, respectively. This is the first time that such technological devices are being used in a real-world study of asthma at a country-level in Greece. Thus, the novelty of EMPOWAIR extends beyond the standard measures for addressing drug effectiveness in a traditional clinical care setting and is enhanced by its specific decentralized design, which employs a hybrid (remote and on-site) patient monitoring and data collection methodology. Importantly, the specific PROs and device-derived outcome measures used in EMPOWAIR were selected based on patient input that was received through patient focus groups conducted by a third party on behalf of the study sponsor.
The patient’s perception of asthma symptoms and the impact of the disease on various aspects of HRQoL and daily activities is increasingly recognized as a valuable tool in assessing asthma symptom control. Such instruments are widely used both in routine clinical practice and clinical trial settings, while the 2022 GINA report recommends patient-reported symptoms as critical components to consider when making treatment decisions and monitoring response to treatment1. The integration of PROs into the workflow of routine care is considered key in a disease like asthma, displaying substantial heterogeneity both in terms of clinical presentation and the underlying pathogenetic mechanisms at play.
On the other hand, using novel technologies such as portable spirometers and WATs is gaining popularity as a reliable and objective tool to monitor asthma in real-time. The protocol will involve home-based spirometry via a novel ultra-portable device which operates through a smartphone or tablet and is suitable for unsupervised use. In general, several studies have demonstrated the high correlation between FEV1 measurements obtained using handheld spirometry devices and in-clinic spirometers and highlight the benefit of having frequent ‘snapshots’ of the patient’s respiratory status as opposed to the once every several months in-clinic spirometric evaluation25. Moreover, a descriptive longitudinal study assessing the quality of home spirometry performed with the Air Next spirometer demonstrated reproducible, high-quality measurements, with the quality grade in 84% (453/541) of sessions classified as at least ‘good’ (i.e. yielding ≥2 acceptable tests with repeatability ≤200 mL) in the unsupervised home setting26. Similarly, there is an increasing pool of publications reporting the benefit of personal WATs to monitor physical activity in patients with asthma27. Such tools are particularly important in severe asthma, as these patients are generally considered a ‘low-active’ population, engaging in considerably low levels of physical activity28. The device used in the study has demonstrated dependability, durability, and acceptability, which render it a valid research tool to accurately measure active minutes of physical activity over 7-day periods29. For the purposes of the study, step counts and active minutes will be recorded, with the latter defined as the minutes spent for activity equivalent to at least 3 metabolic equivalents for task (MET) (i.e. including moderate-intensity level of 3–6 MET and vigorous-intensity level of ≥ 6 MET).
Both of the aforementioned devices are thought to empower patients in their everyday asthma self-management with no intention to interfere with clinicians’ decision-making and patient treatment plan. Two key elements that are essential for ensuring the validity of the relevant device-derived outcomes are patient education and active engagement in these tasks. Towards this goal, patients will be trained by the physicians on the use of both devices at enrollment, emphasizing on the significance of their proper and consistent use, while the study staff will encourage patient engagement through regular reminders and follow-up.
It is generally acknowledged that remote and constant patient monitoring offers additional benefits, especially in the COVID-19 era, as this approach may lower both the frequency and the duration of on-site visits needed and, consequently, the infection exposure risk while, at the same time, the physician can still make informed and timely decisions on the patient’s care. In a broader context, the use of mobile technology allows the patient healthcare plan to be structured around the patients’ own environments, being less burdensome, decreasing barriers and increasing access to healthcare, supporting more timely care with real-time data collection, and increasing convenience for patients.
Limitations
One possible limitation of the study, that could affect the estimation of change in patients’ physical activity levels following benralizumab initiation, stems from a potential for the baseline values to be ‘inflated’ as a result of ‘positive persuasion’ by the physicians. Patients recently given instructions to exercise and, at the same time, excited with a new WAT may be more willing to exercise than they normally would. This may result in higher-than-normal baseline exercise levels, but the enthusiasm and motivation may wane over time, and exercise levels may decrease, irrespective of the treatment, thus leading to a potential underestimation of its effect. Since this bias is inherent in the study design, the step taken to at least partly control for it, is that encouragement and motivation from their physician to use the WAT will take place before measurements used for the analysis are collected. An additional source of bias in this measure stems from the fact that the week during which the measurements used in the analysis are made may not represent a typical period for the patient for various reasons; to appraise this bias, information on whether this was a typical week for the patients, or if there were any non-asthma related reasons that prevented them from exercising as they wished, will be collected in a diary and recorded in the eCRF.
In the absence of a control group, it will be difficult to account for factors that could impact on the primary outcome, including but not limited to seasonal variations of asthma triggers, such as viral infections and environmental allergens, owing to the time-varying recruitment rates among the participating sites. For instance, given that the primary endpoint will be assessed at 16 weeks post-baseline, patients enrolled in the study in spring are expected to have better outcomes than those starting in fall, as the prevalence of viral infections is much lower in the country during the summer. In addition, it will be also difficult to ascertain any positive effects derived from the practices applied in the study from the benefit attributed to the treatment itself, since empowering and motivating patients to take co-responsibility for the management of their condition may enhance treatment outcomes and patient satisfaction. Furthermore, without a control group, attrition bias, deriving from patients who experience smaller or no benefit from the treatment being more likely to discontinue participation, cannot be excluded. In contrast, patients deriving greater benefit may feel that it is worth the effort and be more actively engaged in the proper use of the devices. Such effects and the impact of potential confounders, as those mentioned above, will be evaluated in the context of the analysis with the implementation of appropriate statistical methodologies. There is also a likelihood for information bias impacting the outcomes employing home-based spirometry assessments and WAT-derived activity measurements, originating from the fact that for patients initiating benralizumab at enrollment or within 7 days from enrollment, baseline measurements will be obtained after benralizumab initiation. Nonetheless, it is considered that this time delay is too short to substantially affect the robustness of these outcomes.
Despite the aforementioned limitations, as well as others that are inherent to its observational design, the EMPOWAIR study is anticipated to yield valuable RWE, which is undoubtedly more representative of both the study population of interest and outcomes under observation compared to randomized controlled clinical trials that provide information on a selected group of patients chosen on the basis of strict and narrow eligibility criteria and treated in a controlled manner. With regard to the external validity, the generalizability of the study outcomes will be enhanced by enrolling patients from geographically diverse locations throughout Greece, accounting for variations in medical practice paradigms. Nevertheless, since the study sites will be selected through non-probability sampling and patients are required to be technologically literate in order to qualify for study participation, the generalizability of the study results will be indeterminate.
CONCLUSIONS
Evidence generated in EMPOWAIR is anticipated to shed multidimensional insights on the effects of benralizumab treatment on HRQoL, physical and emotional well-being, and respiratory indices in patients with SEA treated in routine care settings in Greece. Focusing on the patient, the study will utilize remote patient monitoring through advanced technologies that promise to empower patients to better manage their disease and actively participate in their healthcare plan.