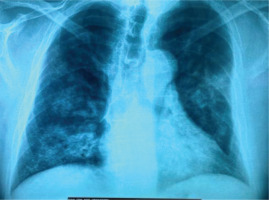
A 62-year-old man presented in the emergency department with fever and dry cough. He was a current smoker (20 pack-years), obese (ΒΜΙ: 43 kg/m2), and he started treatment for benign prostate hyperplasia following an episode of macroscopic hematuria about two months ago (unavailable laboratory tests at that time). No other past medical history was reported. Physical examination on admission revealed crackles in lung lower parts. PCR test for SARS-CoV-2 was negative. Blood tests showed increased C-reactive protein, anemia, and hypoalbuminemia, while arterial blood gas analysis displayed hypoxemia (pO2: 69 mmHg, pCO2: 36 mmHg, pH: 7.47, HCO3: 26 mmol/L). The chest X-ray showed infiltrates in both lungs (Figure 1). He was admitted at the Respiratory Department, and he was treated with antibiotics with a provisional diagnosis of community acquired pneumonia. In less than 24 hours after admission, he experienced hemoptysis. A thorough work-up was initiated measuring urine dipstick, which showed proteinuria and hematuria, and blood autoantibodies titers were ordered. Chest CT scan findings included ground glass opacities (GGOs) and crazy paving; and patchy GGOs associated with smooth septal thickening. Additionally, ‘air bronchogram’; visualization of bronchial structures containing air in the context of consolidation areas, and ‘dark bronchus sign’; and darker bronchus in the context of GGOs, are shown (Figure 2). These findings indicate hemorrhagic alveolitis or edema, thus the provisional diagnosis was alveolar hemorrhage. Vasculitis was put forward as the most probable diagnosis, as those abnormalities, along with the bilateral, para-hilar distribution (Figure 2), are common in diffuse alveolar hemorrhage associated with vasculitis. Therefore, the patient was to be subjected to plasma exchange. At that time he was rapidly deteriorating, experiencing massive hemoptysis and severe respiratory failure, and was transferred to the intensive care unit. The patient was intubated but did not manage to be stabilized and died a few hours later. The next day, serology revealed high titer of MPO/p-ANCA antibodies and the final diagnosis was ANCA-associated vasculitis with lung and renal involvement.
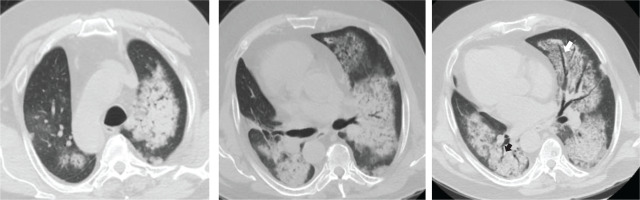
Figure 2
Thorax CT, axial plane showing ground glass opacities, crazy paving, patches of consolidation with ‘air bronchogram’ (black arrow) and ‘dark bronchus sign’ (white arrow), consistent with diffuse alveolar hemorrhage
Herein, we described a patient who presented with symptoms typical for pneumonia, who deteriorated a few hours after admission. The rapid progression and the presence of hemoptysis led to a rethink of the provisional diagnosis.
ANCA-associated vasculitis (AAV) represents systemic, small-vessel vasculitis and is characterized by the loss of tolerance to neutrophil antigens and the development of autoantibodies to the neutrophil proteins MPO and PR3. They are considered to be rare with a prevalence of 46–184 per million people1. Lung involvement is well described in ANCA-associated small vessel vasculitis2, and a recent study found that the majority of patients with ANCA-associated small vessel vasculitis, who were admitted with acute respiratory failure, experienced diffuse alveolar hemorrhage3. However, it is often challenging for the physician to detect small vessel vasculitis in a patient without any known past medical history, who is presenting with lung infiltrates and fever4. In our case, the quick diagnosis in less than 48 hours was crucial, given the rapid deterioration and the need for intubation. Conclusively, it should be highlighted that in patients presenting with pulmonary infiltrates and evidence of renal dysfunction, diffuse alveolar hemorrhage in the context of systemic vasculitis should be suspected.