INTRODUCTION
Since the beginning of the COVID-19 (coronavirus disease 2019) pandemic, patients with chronic respiratory diseases were considered a possible high-risk group for severe disease and negative outcomes. Asthma and chronic obstructive pulmonary disease (COPD) are the most common obstructive respiratory diseases. Asthma is a heterogeneous disease of the airways which affects globally people of any age and race, with a prevalence that ranges to almost 320–350 million patients1, while COPD is a progressive disease characterized by persistent airflow limitation and chronic inflammation that causes remodeling of the airways and the parenchymal structures, with a prevalence estimated to be almost 6% of the global population2.
The severity of both asthma and COPD may vary from very mild, in which there is no need for maintenance treatment, to very severe which influences negatively the patients’ health-related quality of life. Patients with obstructive lung diseases experience exacerbations which require special treatment and are most often caused by viral infections3. Accordingly, in the era of SARS-CoV-2 pandemic, it is of great interest to elucidate the link – if any – between obstructive pulmonary diseases and SARS-CoV-2 infection in terms of prognosis, disease severity and survival.
Current literature data show that asthma prevalence among hospitalized patients with COVID-19 is no higher compared to the general population4. This comes in contrast with hospitalized patients with influenza in which asthma prevalence is much higher. This is the reason why asthma is no more considered as a risk factor for severe COVID-19 whereas it has been even supported that it could act protectively5-7. Moreover, so far, a clear correlation between asthma and worse outcomes from SARS-CoV-2 infection has not been demonstrated. A meta-analysis that assessed potential predictors of mortality and included 27 observational studies, demonstrated that asthma was not associated with increased risk of severe disease or death from COVID-19 in contrast to other diseases including COPD8.
On the other hand, it is known that COPD patients are at high risk of mortality from respiratory infections, such as influenza and community acquired pneumonia (CAP)9,10. However, the prevalence of COPD among COVID-19 patients was lower than expected11. This paradox can be explained by the fact that COPD patients, similarly to patients with other respiratory or smoking-associated conditions, have been shielded during the pandemic, and thus less exposed to pathogens. Previous studies have shown that approximately 1.2% of patients with underlying COPD were identified with severe COVID-19, while 29% of the hospitalized patients with COPD died from COVID-1912. Furthermore, it has been shown that COPD constitutes a risk factor for poor COVID-19 outcomes13,14 and a high mortality risk in COVID-19 patients15,16.
Although in recent years several studies have evaluated the relation between obstructive lung diseases and COVID-19, there are still several unanswered questions. According to the above, the aim of the present study was to describe the epidemiological and clinical characteristics of patients admitted to the ICU for severe COVID-19 infection, to evaluate differences compared to patients with severe COVID-19 without obstructive lung disease, and to identify possible risk factors related to poor outcomes.
METHODS
Study design
This was a retrospective analysis of prospectively collected data conducted in the Intensive Care Unit of the 1st Respiratory Department of the National and Kapodistrian University of Athens in Sotiria Chest Diseases Hospital between 27 August 2020 and 10 November 2021. Consequent patients with confirmed COVID-19 who were admitted in the ICU were included in the study. Patients from the same cohort have been already used in a previous study of our department17. Patients who required hospitalization in the ICU for <48 hours, those transferred from another ICU after a prolonged length of ICU stay, those without a fully available medical history and those with respiratory disease other than asthma or COPD, were not included in the study.
Epidemiological characteristics including age, gender, body mass index (BMI), smoking history, comorbidities, disease severity scores (APACHE II, SAPS3, SOFA), manifestations of disease (onset of symptoms, date of hospital admission, date of ICU admission, PaO2/FiO2 (PF) ratio, laboratory findings, intubation, type of respiratory support, length of mechanical ventilation), medication/treatment [dose of corticosteroids, remdesivir, tocilizumab, anakinra, anticoagulation), prone position, continuous renal replacement therapy (CRRT)], and outcome data (barotrauma, thromboembolism, septic shock, infections, survival, length of hospital or ICU stay, length of mechanical ventilation) were recorded.
The study was conducted in accordance with the Declaration of Helsinki. The analyses results were obtained exclusively from routine clinical practice in accordance to the clinician’s prescription at the sample time. The clinical management of patients was not affected. No analyses were performed on stored samples.
Evaluation of comorbidities
Major comorbidities were recorded in each patient. The Charlson comorbidity index (CCI) was used in order to evaluate the comorbidities severity and to assess their additive influence in the patients’ general health status18.
Evaluation of COVID-19 severity
Evaluation of severity upon admission to the ICU was recorded based on the following scores: APACHE II, SAPS3 and SOFA19-21.
Evaluation of arterial blood gases
In all patients, arterial blood samples were taken for the measurement of PaO2 and PaCO2 using a commercially available blood gas analyzers on days 1, 4, 7, 10 and 17 during ICU stay and PaO2/FiO2 (PF) ratios were calculated.
Statistical analysis
Normality of distributions was checked with Kolmogorov-Smirnov test. Comparisons between patients with and without obstructive lung disease were performed with Mann-Whitney U-tests for skewed variables and with unpaired Student’s t-tests for normally distributed variables. For categorical data, comparisons between groups were performed using chi-squared tests. Correlations were assessed using Spearman’s rank correlation coefficient for skewed variables and Pearson’s correlation coefficient for normally distributed variables. Group data are expressed as mean ± standard deviation or as median (interquartile range) for normally distributed and skewed data, respectively.
Overall survival time was calculated from admission to ICU until death. Patients discharged alive from the hospital were right censored at the date of exit. Kaplan-Meier estimates and log rank tests were used to describe and visualize the effect of categorical variables on survival. Cox proportional hazards models were used to explore the prognostic value of covariables. A p<0.05 was considered statistically significant. Analysis was performed using the SPSS 23 statistical package (SPSS, Chicago, IL).
RESULTS
Characteristics of critically ill COVID-19 patients
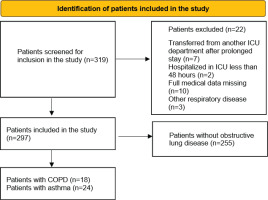
Between 27 August 2020 and 10 November 2021, 319 patients were admitted to the ICU. Patients initially screened and those included and excluded from the study are shown in Figure 1. In all, 22 were excluded from this study: 7 were transferred from another ICU after a prolonged stay there, full data were missing for 10 patients due to transfer out of hospital, 2 were hospitalized in the ICU for <48 hours, and 3 patients were excluded due to respiratory disease other than asthma or COPD. Finally, 297 patients were included in the study. Regarding time of disease, patients were distributed in the following pandemic waves: 93 patients in the second wave (August 2020– December 2020), 185 patients in the third wave (January 2021–August 2021) and 22 patients in the fourth wave (September 2021–November 2021).
In our population, 85 patients were females (28.6%) and 212 (71.4%) males. The median age was 62 years (IQR: 51–70) and their median BMI (kg/m2) was 29.3 (26.1–33). Among them, 11 (3.7%) patients were additionally supported with extra corporeal membrane oxygenation (ECMO). The median PF ratio on admission day was 103.4 (78.2–134.7) and the median (IQR) of APACHE, SOFA and SAPS3 were 11 (8–14), 3 (2–6), and 43 (38–51), respectively. All patients received dexamethasone due to respiratory failure on standard (6 mg × 1 iv for 10 days) or higher dosage (>6 mg × 1 iv for >10 days, usually 20 mg). Due to the studied period only a small minority of patients was vaccinated (12 patients in total of whom 1 had asthma). Demographics and medical characteristics of the study participants are presented in Tables 1 and 2.
Table 1
Clinical characteristics of asthma and COPD patients
[i] ACE/ARB: angiotensin converting enzyme inhibitors/angiotensin recepto blockers. APACHE II: acute physiology and chronic health evaluation II. BMI: body mass index. COPD: chronic obstructive pulmonary disease. ICU: intensive care unit. IQR: interquartile range. PF: PaO2/FiO2. SAPS3: simplified acute physiology score 3. SOFA: sequential organ failure assessment.
Table 2
Treatment and outcomes of asthma and COPD patients
| Total (N=297) n (%) | Control group (N=255) n (%) | Asthma (N=24) n (%) | p | COPD (N=18) n (%) | p | |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| High dose corticosteroids | 116 (39.1) | 98 (38.4) | 14 (58.3) | 0.057 | 5 (27.8) | 0.367 |
| Tocilizumab | 81 (27.3) | 77 (30.2) | 2 (8.3) | 0.023 | 2 (11.1) | 0.084 |
| Remdesivir | 241 (81.1) | 208 (81.6) | 19 (79.2) | 0.773 | 14 (77.8) | 0.69 |
| Anakinra | 22 (7.4) | 19 (7.5) | 2 (8.3) | 0.876 | 1 (5.6) | 0.765 |
| Anticoagulation at hospital | 0.084 | 0.767 | ||||
| No | 1 (0.3) | 1 (0.4) | 0 | 0 | ||
| Prophylaxis | 232 (78.1) | 194 (76.1) | 23 (91.7) | 15 (83.3) | ||
| Full | 64 (21.5) | 60 (23.5) | 1 (4.2) | 3 (16.7) | ||
| CRRT | 67 (22.6) | 53 (20.8) | 2 (8.3) | 0.143 | 12 (66.7) | <0.001 |
| Prone | 104 (35) | 88 (34.5) | 8 (33.3) | 0.908 | 8 (44.4) | 0.394 |
| ECMO | 11 (3.7) | 10 (3.9) | 0 | 0.323 | 1 (5.6) | 0.733 |
| Adverse events/outcomes | ||||||
| Barotrauma | 44 (14.8) | 38 (14.9) | 3 (12.5) | 0.751 | 3 (16.7) | 0.839 |
| Thromboembolism | 33 (11.1) | 30 (11.9) | 3 (12.5) | 0.926 | 0 | 0.132 |
| Hyperglycemia | 115 (38.7) | 100 (39.5) | 8 (33.3) | 0.552 | 7 (38.9) | 0.893 |
| Septic shock | 93 (31.3) | 74 (29.2) | 6 (25) | 0.661 | 13 (72.2) | <0.001 |
| HAP/VAP, n/m (%)* | 67/210 (31.9) | 57/183 (31.14) | 5/18 (27.8) | 0.768 | 5/9 (55.6) | 0.126 |
| Fungal infections | 22 (7.4) | 18 (7.1) | 1 (4.2) | 0.591 | 3 (16.7) | 0.139 |
| Positive culture | ||||||
| Blood | 65 (21.9) | 54 (21.2) | 3 (12.5) | 0.314 | 8 (44.4) | 0.023 |
| Central line | 37 (12.5) | 32 (12.7) | 3 (12.5) | 0.972 | 2 (11.1) | 0.906 |
| Bronchial secretions | 132 (44.4) | 108 (42.5) | 11 (45.8) | 0.754 | 13 (72.2) | 0.014 |
| Urine | 63 (21.2) | 51 (20.3) | 8 (33.3) | 0.138 | 4 (22.2) | 0.751 |
| Hospitalization (days), median (IQR) | ||||||
| Length of ICU stay | 10 (6–23) | 10 (6–23) | 12.5 (5.8–19.3) | 0.833 | 16.5 (8.3–47) | 0.147 |
| Length of mechanical ventilation | 14 (8–38.5) | 12 (6–35) | 16 (10–35.5) | 0.361 | 14 (7–51) | 0.443 |
| Length of hospital stay | 26 (17–40.3) | 26 (18–40) | 30.5 (18.5–41.8) | 0.748 | 23.5 (15–56.3) | 0.753 |
| In-hospital stay prior to ICU | 3 (1–5) | 3 (1–5) | 3 (1–5) | 0.841 | 2.5 (1–7) | 0.702 |
| 28-day mortality | 47 (15.8) | 38 (14.9) | 1 (4.2) | 0.147 | 8 (44.4) | <0.001 |
| Exit ICU | 217 (73.1) | 192 (75.3) | 20 (83.3) | 0.378 | 5 (27.8) | <0.001 |
| Exit Hospital | 212 (71.4) | 188 (73.7) | 20 (83.3) | 0.302 | 4 (22.2) | <0.001 |
Among the 297 patients, 42 patients had an obstructive pulmonary disease: 24 asthma (8.1%), and 18 COPD (6.1%). The characteristics of each group are further discussed in the next sections.
Asthma patients’ characteristics
Twenty-four patients had a history of asthma. None of them was classified as severe asthma and none was under biologic agent treatment. Ten patients were females (41.7%) and fourteen (58.3%) males. The median age was 50.5 years (44–61.5) and their median BMI was 29.95 (26.5–37.2). Twenty patients (83.3%) with asthma never smoked, while there were three current smokers (12.5%) and one ex-smoker (4.2%). All the patients were under standard treatment with inhaled corticosteroids (ICS). None of the patients had an exacerbation of asthma or admitted to the ICU due to their asthma. The median PF ratio on admission day was 110.5 (84.8–131.2) and the median (IQR) of APACHE II, SOFA and SAPS3 were 9 (7–13), 2.5 (2–5.75) and 38 (36–45), respectively.
Comparing asthma patients with the ICU patients without obstructive airways disease (ICU-non OAD) group, asthma patients were younger (50.5 vs 62 years, p=0.003), usually never smokers (83.3% vs 58.8%, p=0.032) and had less comorbidities with a lower median CCI index (1 vs 2, p=0.006). The most common comorbidity was arterial hypertension. The severity on admission was less based on SAPS3 index (38 vs 43, p=0.015), while no statistically significant differences were identified with APACHE, SOFA and PF ratios on days 1, 4, 7, 10 and 15.
All asthma patients received dexamethasone due to respiratory failure and more frequently a high regimen (dosage equal to 20 mg dexamethasone) although this was not statistically significant (58.3% vs 38.4%, p=0.057). Compared to the ICU-non OAD group, asthma patients received less frequently tocilizumab (8.3% vs 30.2%, p=0.023).
Eleven patients were intubated (45.8%) of whom eight arrived intubated (33%) and three (18.8%) were intubated in the ICU setting, while in the ICU-non OAD group 149 out of 255 (58.4%) patients required intubation. None of the asthmatic patients needed ECMO support. No differences with the ICU-non OAD group were identified regarding the need for mechanical ventilation.
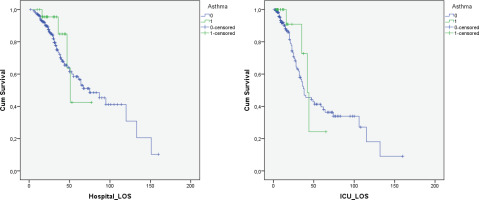
The median length of ICU stay of patients with asthma was similar to the ICU-non OAD group (12.5 vs 10 days, p=0.833), as well as the median length of hospital stay (30.5 vs 26 days, p=0.748). Duration of mechanical ventilation for intubated patients was also similar (16 vs 12 days, p=0.361). The 28-day mortality was lower for asthmatic patients compared to the ICU-non OAD group but without statistical significance (4.2% vs 14.9%, p=0.147). In addition, Kaplan Meier survival analysis for hospital and ICU survival showed no statistically significant difference between asthma patients and the ICU-non OAD group (0.467 and 0.578, respectively) (Figure 2). Cox regression analysis was performed for the variables age, gender, BMI, CCI, SAPS3, asthma, and COPD. Asthma was not correlated to ICU mortality in the univariate analysis (HR=0.688; 95% CI: 0.251–1.887, p=0.468). CCI and SAPS3 were found to be independent predictors of survival (Table 3).
Table 3
Cox regression analysis
Figure 2
Hospital survival of asthma patients in comparison to control group showed no difference [Log Rank (Mantel-Cox) p=0.467]. ICU survival of asthma patients in comparison to control group showed no difference [Log Rank (Mantel-Cox) p=0.578]
Regarding the other adverse events (barotrauma, thromboembolism, hyperglycemia, septic shock, hospital/ventilator associated pneumonia, fungal infections, positive cultures) no differences were identified compared to the ICU-non OAD group.
Laboratory tests on ICU admission for patients with asthma showed statistical differences compared to the ICU-non OAD group for urea, creatinine, SGOT, troponin, ferritin, and IL-6, all of which were lower in asthmatics (Table 4).
Table 4
Laboratory tests of COPD and asthma patients on admission in ICU
COPD patients’ characteristics
Eighteen patients had a diagnosis of COPD. Two patients were females (11.1%) and sixteen (88.9%) males. The median age was 71.5 years (66.5–76.8) and their median BMI was 28.35 (15.8–31.7). One patient (5.6%) with COPD never smoked (emphysema of unknown etiology), while there were four current smokers (22.2%) and thirteen ex-smokers (72.2%). The median PF ratio on admission day was 96.6 (78.3–121.8) and the median (IQR) of APACHE, SOFA, and SAPS3 were 15 (11–17), 6.5 (2.8–8.3), and 60.5 (51–62.5), respectively.
Comparing COPD patients with the ICU-non OAD group, COPD patients were older (71.5 vs 62 years, p<0.001), more commonly smokers (current or former) (94.4% vs 41.2%, p<0.001)) and had more comorbidities with a higher CCI (5 vs 2, p<0.001). Diabetes was more common (50% vs 21.2%, p=0.005). The rest most frequent comorbidities were hypertension and dyslipidemia. One third of the patients were under anticoagulation/antiplatelet treatment at home (38.9% vs 22.7%, p=0.151). The severity on admission was worse based on SAPS3 index (60.5 vs 43, p<0.001) and SOFA (6.5 vs 2, p=0.008), while no significant difference was identified with APACHE and PF ratios on admission or on day 7, 10 and 15. PF ratio on day 4 was lower (106.25 vs 134.05, p=0.014).
All patients received dexamethasone due to respiratory failure and no differences to the ICU-non OAD group were identified regarding medical treatment (dosage of dexamethasone, tocilizumab, remdesivir, anakinra, anticoagulation). COPD patients needed continuous renal replacement therapy more frequently (66.7% vs 20.8%, p<0.001).
Only one out of 18 (5.6%) patients with COPD was not intubated, while the rest required mechanical ventilation. COPD patients were more frequently intubated in the ward and arrived intubated in ICU (77.8% vs 43.9%, p=0.005). For non-intubated patients on ICU arrival, it was more common to be intubated in the ICU (75 vs 25.9%, p=0.029) and less frequent to be never intubated (5.6% vs 41.6%, p=0.002). One patient (5.6%) was further supported with ECMO (extra-corporeal membrane oxygenation).
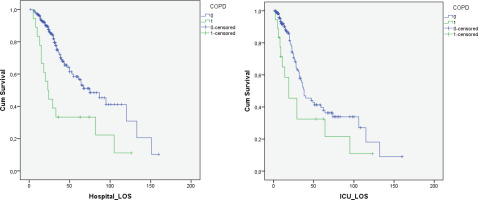
The median length of ICU stay of patients with COPD was similar to the ICU-non OAD group (16.5 vs 10 days, p=0.147), as well as the median length of hospital stay (23.5 vs 26 days, p=0.753). Duration of mechanical ventilation for intubated patients was also similar (14 vs 12 days, p=0.443). The 28-day mortality was higher for COPD patients compared to the ICU-non OAD group (44.4% vs 14.9%, p<0.001). In addition, Kaplan Meier survival analysis for hospital and ICU survival showed statistically significant reduced survival for COPD patients (0.001 and 0.039, respectively) (Figure 3). COPD patients had a lower chance to exit the ICU (27.8% vs 73.3%, p<0.001) and the hospital (22.2% vs 71.7%, p<0.001) compared to the ICU-non OAD group. Cox regression analysis was performed for the variables age, gender, BMI, smoking, CCI, SAPS3, asthma, and COPD. Although in the univariate analysis COPD was correlated to ICU mortality (HR=1.873; 95% CI: 1.029–3.411, p=0.04), after adjusting for age, CCI and SAPS3 using a Cox Proportional Hazards model, COPD was not found to be a predictor of ICU mortality risk (AHR=0.958, 95% CI: 0.375–2.445). CCI and SAPS3 were found to be independent predictors of survival (Table 3).
Figure 3
Hospital survival of COPD patients in comparison to control group showed worse survival curve for COPD patients [Log Rank (Mantel-Cox) p=0.001]. ICU survival of COPD patients in comparison to control group showed worse survival curve for COPD patients [Log Rank (Mantel-Cox) p=0.039]
COPD patients had more frequently septic shock (72.2% vs 29.2%, p<0.001), positive bronchial secretion cultures (72.2% vs 42.5%, p=0.014) and positive blood cultures (44.4% vs 21.2%, p=0.023). The rest of recorded adverse events (barotrauma, thromboembolism, hyperglycemia, hospital/ventilator associated pneumonia, fungal infections) were no different to the ICU-non OAD group.
Laboratory tests on ICU admission for patients with COPD showed statistical differences compared to the ICU-non OAD group (lower hemoglobin, higher d-dimers, higher urea, higher creatinine) (Table 4).
DISCUSSION
In our study, the prevalence of asthma and COPD in critically ill COVID-19 patients was similar to that described in the literature (8.1% and 6.1%, respectively)4,11. However, we demonstrated that patients with obstructive pulmonary diseases have some distinctive characteristics.
Asthma patients are younger with less comorbidities and possibly less severe disease based on SAPS3 score. Aveyard et al.22 showed that although asthma patients are rarely admitted in the ICU and do not show an increased risk for ICU admission (HR=1.08; 95% CI: 0.93–1.25), severe asthma patients might be at higher risk after adjusting for confounding factors (AHR=1.3; 95% CI: 1.08–1.58). However, in our population none of the patients had a diagnosis of severe asthma.
In our study, asthma patients received higher dosages of dexamethasone and less frequently tocilizumab. The differences in dosages of dexamethasone could be possibly attributed to the overall tendency to treat asthma patients with steroids. Intubation rates were similar to the ICU-non OAD, a fact that is in concordance with the literature23. Mortality was found to be similar with the ICU-non OAD which is also in line with published data22,24. Interestingly, none of the asthma patients had an exacerbation in our population. Indeed, as shown in the literature, COVID-19 rarely seems to be a trigger for asthma exacerbation4.
Unfortunately, there are no data about the specific phenotypes of asthma in our population. Nevertheless, different asthma phenotypes and individual risk factors should be also considered in future studies, as the severity of asthma among COVID-19 patients may differ. Until now, several studies that assessed the outcome of COVID-19 infection in asthmatics receiving different treatments found no statistically significant differences in mortality between patients receiving ICS/LABA, OCS or patients receiving biologic therapies with monoclonal antibodies25,26. The situation is not yet clarified, since a real-life study from Israel which evaluated over 80000 asthmatics showed that systemic OCS use is not a risk factor for getting infected, whereas it remains an important prognostic factor for more severe COVID-19 disease and all-cause mortality. Biologics on the other hand express a better safety profile in terms of COVID-19, with infection and mortality rates similar to those reported in mild-moderate asthmatics27. ICS have been associated both with more and less favorable outcomes in several studies.
Analyzing the laboratory tests on admission in ICU in our cohort, no difference in eosinophils was identified. This is in line with published data showing that there is no association between severe COVID-19 disease and biomarkers signifying type 2 inflammation, such as blood eosinophil count, atopy, or history of allergic rhinitis28. Another study from the USA, including around 10000 infected patients of whom 4.4% were asthmatics, demonstrated that patients with asthma and especially those with more than 200 eosinophils/mL in their blood count irrespective of asthma history had lower hospitalization, ICU admission and mortality rates compared to the ICU-non OAD29. It should be noted that upon admission our patients had already been treated with corticosteroids, therefore the possible increase in eosinophils could not have been reported even if it existed.
On the other hand, patients with COPD are older with more comorbidities – especially diabetes – and seem to have more severe disease on admission based on SAPS3 and SOFA. Similarly in the literature, patients with COPD and severe COVID-19, who are admitted in the ICU, are more likely to be men, of older age (>65 years) and have a longer history of COPD30. Furthermore, COPD patients often suffer from other comorbidities as well, which have an additional negative impact on the final outcome.
COPD patients were also found to be more commonly supported by mechanical ventilation and continuous renal replacement therapy. Researchers in South Korea studied COVID-19 patients with and without COPD, in order to estimate its impact in respiratory failure and mortality. Patients with COPD required admission to the ICU and mechanical ventilation in greater proportions compared to the population without COPD24. Numerous studies arrived at similar conclusions31,32. Cornwell et al.33 compared hospitalized patients with COPD and COVID-19, with patients with COPD without COVID-19. Risks of ICU admission, invasive mechanical ventilation, and death not only were high among patients with COPD and COVID-19, but also exceeded the corresponding risks among patients with COPD without COVID-1933. In a meta-analysis by Jain et al.34, 1813 COVID-19 patients with severe disease were included. Among these patients, COPD was the strongest predictive comorbidity (followed by cardiovascular disease and hypertension) for severe disease and admission to the ICU.
The 28-day survival of COPD patients in our study and the overall survival (exit from ICU or hospital) were worse in univariate analysis. However, after correction for possible confounding factors the difference in survival/mortality was not sustained. This could possibly raise the question whether COPD on its own or the multiple risk factors that this group of patients share, are the main responsible factors for worse survival. Grasseli et al.35 in their cohort study included 3988 critically ill patients, from Lombardy in Italy, with confirmed diagnosis of COVID-19, who were admitted to the ICU. COPD was found to be an independent risk factor associated with mortality. A large cohort, including over 8 million participants, demonstrated that COPD is associated with worse outcomes from COVID-19 after adjusting for confounding factors22. In contrast, Marron et al.36 also noted that hospitalized patients with COVID-19, who suffered from COPD, had not a worse outcome compared to patients with other comorbidities.
Other adverse events were also more common in our COPD group: septic shock, positive culture of bronchial secretions, and positive blood cultures. In the literature it is noted that secondary infections are more common in these patients because of the colonization of the respiratory tract with pathogenic bacteria37,38. Although barotrauma could be expected theoretically to be more common due to the frequently emphysematic lung parenchyma or the overdistention of COPD (or asthma) patients, such a difference was not demonstrated. Few data exist on the literature about barotrauma and the role of obstructive pulmonary diseases. In a systematic review by Chong et al.39, <30% of barotrauma patients had a history of pre-existing lung diseases and only the minority of patients were current or former smokers.
Limitations
Our study has several limitations. First of all, patients with severe asthma have not been included in the study. This is mainly due to the fact that during the COVID pandemic, it has been observed that severe asthmatics did not experience severe disease and were not at higher risk for severe complications. Thus, the representation of this group of patients in our cohort is poor and not able to lead to any conclusions40. Furthermore, the results in the COPD population cannot be stratified by disease severity class since no data regarding spirometry were available in our patients. This fact probably means that our observation regarding adverse outcomes and severe complications concern all COPD patients and not those with more severe disease.
CONCLUSIONS
Comparing asthma and COPD patients reveals that chronic obstructive pulmonary diseases are a wide umbrella that covers quite different groups of patients. Asthma patients do not seem to be at increased risk in comparison with the ICU-non OAD regarding severe COVID-19. COPD patients may not be at higher risk for developing COVID-19 disease, but they most likely have increased risk of poor outcomes when they develop COVID-19, as was shown in our study as well. Physicians should be very willing to advice patients with obstructive pulmonary diseases to take precautions, vaccinate against COVID-19, and remain vigilant.