INTRODUCTION
Pleural effusion represents a major cause of pulmonary mortality and morbidity. There are numerous cases of pleural effusion, divided into transudates and exudates based on Light’s criteria1. Most common diagnoses of transudates are heart failure, cirrhosis and nephrotic syndrome, whereas parapneumonic effusion, cancer, tuberculosis and pulmonary embolism should be taken under consideration regarding exudates2.
Non-small cell lung carcinomas are usually associated with exudative malignant pleural effusions (MPE). Furthermore, the presence of MPE in such patients is associated with poor prognosis and less therapeutic options, as cancer is considered metastatic and scores M1 at the TNM staging system3. The main presenting symptoms are dyspnoea and cough. The amount of pleural fluid correlates with the severity of dyspnoea, however, up to 25% of patients presenting with pleural effusion are asymptomatic4.
In recent studies, patients with adenocarcinoma presented with MPE at a higher frequency than other types5. Moreover some studies correlate EGFR mutations with MPE6. The final diagnosis is made with biopsy which is either done under CT or U/S guidance or through thoracoscopy.
Black pleural effusion is a rare clinical condition under which may be hiding, amongst others, lung cancer, metastatic melanoma, invasive pulmonary aspergillosis and pancreaticopleural fistula. It is crucial that physicians should be aware of this rare entity7.
In the current case report, we present a patient with a large black-colored pleural effusion.
CASE PRESENTATION
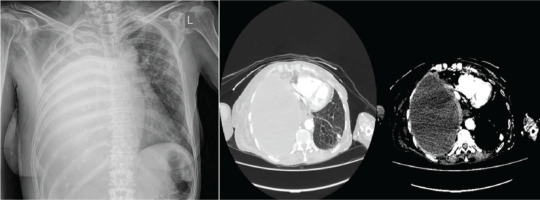
A 68-year-old female patient presented to the Emergency Department due to right-sided pleuritic chest pain and shortness of breath for the past ten days. Also, she reported weight loss (5–6 kg in the past three months) and fatigue. Her personal history includes epilepsy, hypothyroidism osteoporosis, all under treatment and approximately 80 pack-years of smoking. During her presentation in the Emergency Department, she was hemodynamically stable (MAP: 80 mmHg), with SpO2=98%. Her physical examination revealed absence of breath sounds on the right side. Laboratory investigations performed included full blood count, renal-liver function tests, electrolytes, and cardiac and inflammatory markers. The results are reported in Table 1. A chest X-ray and a chest CT were performed, all revealing massive pleural effusion occupying the right hemithorax, diffuse pleural thickening, right lung atelectasis, multiple nodules on left lung, enlarged mediastinal lymph nodes, and subdiaphragmatic extension in contact with the liver as shown in Figure 1. Moreover, CTPA revealed filling defects within the segmental and subsegmental branches of the left pulmonary artery.
Table 1
Characteristics of blood sample
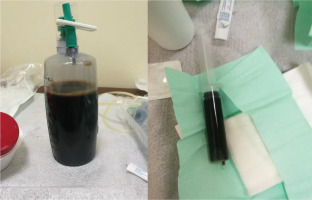
Ultrasound-guided thoracentesis was performed and pleuritic fluid was aspirated. The fluid was macroscopically dark-coloured and viscous (Figure 2). The laboratory analysis showed neutrophilic exudate with low glucose and high lactate dehydrogenase (LDH) (Table 2) according to Light’s criteria: 1) effusion protein/serum protein ratio greater than 0.5; 2) effusion LDH/serum LDH >0.6; and 3) Effusion LDH level greater than two-thirds of the upper limit of the laboratory’s reference range of serum LDH (our laboratory upper limit=250 IU/L). Due to high serum amylase levels (three times the upper limit) together with the presence of subdiaphragmatic effusion, pleuritic fluid amylase was measured. Moreover, due to the massive pleural effusion with contralateral shift, drainage was indicated. At first, a small chest tube was placed (size 10 Fr) which on a daily basis drained around 500 mL for the next 5 days. Subsequently, due to obstruction of the tip of the chest tube, a larger chest tube was placed (size 24 Fr) and approximately 200–300 mL of pleuritic were drained each day. However, the pleural effusion was rapidly regenerated and no improvement was shown in her chest CT. Pleural fluid cultures were negative. As was cytology (three samples 100 mL each). Tuberculosis was ruled out based on low levels of adenosine deaminase (ADA), negative pleuritic fluid culture, combined with negative Ziehl-Neelsen staining of the patient’s sputum.
Table 2
Characteristics of pleural effusion
| Markers | Values |
|---|---|
| Cells (count/μL) | 2000 |
| NEU (%) | 78 |
| LYM (%) | 12 |
| Creatinine (mg/dL) | 0.63 |
| Glucose (mg/dL) | 2 |
| Amylase (mg/dL) | 20922 |
| Total protein (g/dL) | 7.0 |
| Albumin (g/dL) | 3.8 |
| LDH (IU/L) | 5956 |
| ADA (IU/L) | 33 |
As shown in Table 2, due to the high neutrophile count in the pleuritic fluid together with high inflammatory serum markers, empirical antibiotic coverage for parapneumonic effusion was initiated with ampicillin/sulbactam. Moreover, due to respiratory deterioration of the patient in combination with an increase in her inflammatory markers, antibiotic coverage was escalated to piperacillin/tazobactam. In addition, enoxaparin was administrated due to pulmonary embolism.
During her hospitalization, according to her chest CT findings a bronchoscopy was performed, revealing a concentric stenosis of the right upper, middle and lower lobe bronchus. Biopsies were taken using diathermy from the right upper and middle lobe bronchus. Due to thickening of the pleura, fine needle biopsy (FNB) of right pleura in the fifth intercostal space via ultrasound guidance was performed.
The histopathology test from the biopsies of bronchus and the pleura revealed invasive non-mucinous adenocarcinoma of the lung (WHO/2021, Thoracic tumors, 5th Edition). Immunofluorescent investigation showed the following markers: TTF-1 (+100%), Napsin (+100%), CK7(+100%), INSM-1 (-), Chromogranin A (+ in some cancer cells), p40(-), YAP-1 (+), and Ki-67 about 15%. Moreover, the specimens were tested for molecular markers anaplastic lymphoma kinase (ALK) and programmed death-ligand 1(PD-L1), which were negative. Lastly, EGFR control with Idylla EGFR mutation test detected L858R mutation. No other mutation was detected.
Pending histology results, the patient gradually deteriorated with increasing oxygen needs and hemodynamic instability. Sepsis due to catheter related infection from a central venous catheter occurred. Despite supportive therapy the patient succumbed to the disease without receiving oncological treatment.
DISCUSSION
We presented a case of lung adenocarcinoma manifesting as massive pleural effusion of black colour. This unusual colour combined with neutrophilic predominance, high LDH, low glucose and high amylase led us to consider initially a parapneumonic effusion or abdominal pathology.
The black color of malignant pleural effusions suggests accumulation of the fluid for a long time, in contrast to our case where fluid was being rapidly produced. This specific black pleural effusion can be attributed to hemolysis after intrapleural bleeding from lung cancer. Other pathogenetic causes include cytoplasmic melanin production by cancer cells (in metastatic melanoma), black spores of the fungus by Aspergillus niger or necrotic damage by Rhizopus oryzae, necrotic ascitic and pancreatic fluid (in pancreaticopleural fistula)7.
Increased amylase in pleural fluid may be seen not only in pancreatic or esophageal pathology but also in other common conditions such as lung adenocarcinoma, parapneumonic effusion, and tuberculosis8.
A bacterial superinfection of the malignant effusion was also a diagnostic possibility. However, cultures did not grow pathogens while antibiotic coverage did not lead to any improvement in the patient’s clinical picture.
According to the new TNM3, the presence of malignant pleural effusion increases the staging of the tumor to M1a, limiting the available therapeutic options. It is crucial that diagnosis and oncologic treatment are offered promptly in an effort to improve the quality of life and prognosis of such patients.
EGFR mutations in lung cancer offer the possibility for targeted therapy which can significantly prolong survival and be given irrespective of performance status. In addition, a correlation between MPE and EGFR can be found in the literature. More specifically, Chai et al.6 indicate that expression of EGFR-L858R in lung cancer cells results in up-regulation of the C-X-C chemokine receptor type 4 in association with increased cancer cell invasive ability and MPE formation6.
CONCLUSION
In our patient, diagnostic workup and treatment were initially targeted towards an infectious process or an extension of abdominal pathology. However, investigation for malignancy was undertaken in parallel, despite impaired respiratory and performance status. The presence of EGFR L858R mutation in adenocarcinoma cells could have been a most valuable therapeutic option.
Unfortunately, the patient deteriorated rapidly, due to the advanced malignant stage, and no oncologic treatment was offered. An earlier presentation and consequently diagnosis could have offered a chance for targeted therapy and improved prognosis.