INTRODUCTION
The Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), which is the causing agent of coronavirus disease COVID-19, is an extremely infectious viral pathogen accounting for the current pandemic1. Up to 1 November 2021, more than 251 million infections with SARS-CoV-2 were reported globally, with 5 million deaths worldwide attributable to COVID-192. The clinical manifestations of COVID-19 demonstrate high diversity, ranging from asymptomatic infection to acute respiratory distress syndrome (ARDS), and death. Despite being usually asymptomatic, COVID-19 can also present with mild symptoms, taste and smell changes, myalgias, or moderate pneumonia. Occasionally, COVID-19 appears as severe pneumonia and ARDS, primarily affecting individuals with comorbidities and older patients, leading to high mortality3. Cardiovascular disease, chronic respiratory diseases, obesity, diabetes mellitus, chronic kidney disease, severe immunosuppression, hematologic or solid organ malignancy have been associated with disease severity4–6. Moreover, disease progression and worse hospitalization outcomes have been attributed to male sex, advanced age, obesity, being non-White, smoking history, Alzheimer’s disease, Parkinson’s disease, COPD, diabetes, hypertension, malignancy, coronary heart disease, and chronic kidney disease7-9. Currently there is no specific treatment for COVID-19 available and should be principally managed by vaccination, preventing the transmission of the virus by averting the exposure, and smoking cessation10. Previous studies have demonstrated a survival benefit by administering dexamethasone and tocilizumab in patients that require oxygen therapy or invasive mechanical ventilation (IMV), while the duration of hospitalization can be reduced by remdesivir11-13. COVID-19 patients often develop secondary bacterial infections in the course of their hospitalization, especially during ICU stay14.
Up to 1 November 2021, there were more than 745000 SARS-CoV-2 documented infections in Greece resulting in more than 16000 deaths. During the same period in the region of Epirus, northwestern Greece, where the University General Hospital of Ioannina serves as a referral center for patients with COVID-19, 14050 cases and 302 deaths have been confirmed15,16. In the present study, we illustrate the pre-hospitalization baseline characteristics and comorbidities of patients admitted with COVID-19 to the University Hospital of Ioannina during the first 4 waves of the pandemic, and to analyze how these might have influenced the clinical outcomes of these patients.
METHODS
In the present retrospective observational study of all consecutive patients admitted to the COVID-19 infectious diseases units of the University General Hospital of Ioannina (March 2020 – August 2021), subjects’ demographic data were recorded upon their admission including sex, age, body mass index (BMI, kg/m2), treatment prior to admission, comorbidities, and smoking status. Upon admission we recorded additionally clinical parameters; more specifically vital signs, the presence of dyspnea, and the level of respiratory failure.
The following pre-hospitalization parameters were recorded: demographics; date of admission defining in which COVID wave the admission took place; lifestyle risk factors; BMI with obesity being defined as BMI ≥30 kg/m2; cigarette smoking habit; history of comorbidities (i.e. hypertension, COPD, asthma, diabetes, malignancy, atherosclerotic cardiovascular disease); type of drug therapy chronically taken at home (the chronically received medication for past medical history). COVID-19 waves were defined as follows: 1st wave March 2020 – August 2020; 2nd wave September 2020 – December 2020; 3rd wave January 2021 – April 2021; and 4th wave May 2021 – August 2021.
We classified patients in three groups based on their outcomes (discharge, death, and intubation and admission to ICU with subsequent discharge) and compared them with each other. The predictors of these outcomes, along with the length of hospitalization were investigated.
Statistical analysis
We used the Shapiro-Wilk and Kolmogorov-Smirnov tests to evaluate if the data had a normal distribution. The chi-squared or Fisher’s exact tests, as appropriate, were used to analyze categorical values, reported as n (%), while continuous variables were analyzed by Student’s t-test (for independent samples of normally distributed data) or by Mann-Whitney U and Kruskal-Wallis tests (for independent samples of non-normally distributed data). We performed univariate and multivariate binomial logistic regression analyses in order to define the parameters predicting mortality starting from the pre-admission parameters considered in the study (demographics, comorbidities, risk factors, chronic disease medication use). Patients were divided in two groups based on the median duration of hospitalization (11 days) to perform the multivariate analysis of length of hospitalization. All statistical analyses were performed using SPSS for Windows 23.0 (SPSS, Chicago, Illinois), with the level of statistical significance set at p<0.05.
RESULTS
Demographics and baseline characteristics
The mean (SD) age of the 627 study participants was 62.5 (16.6) years (76.5% were aged >50 years) and 270 (43.1%) were female. Nineteen patients (3%) were hospitalized during the 1st COVID wave, 206 (32.9%) during the 2nd wave, 295 (47%) during the 3rd wave and 107 (17.1%) during the 4th wave (Tables 1 and 2). The mean (SD) duration of symptoms before hospitalization was 6.66 (0.20) days, with fever being the most frequent symptom (82.7% of the cases) (Table 1). Upon admission the majority of the patients presented with moderate (32.9%) and severe (42.8%) COVID-19 disease (Table 2).
Table 1
Baseline characteristics of the study participants (N=627)
Table 2
Admissions per COVID-19 wave and hospitalization outcome (N=627)
| Variables | n (%) |
|---|---|
| Admissions COVID-19 wave | |
| 1st | 19 (3.0) |
| 2nd | 206 (32.9) |
| 3rd | 295 (47.0) |
| 4th | 107 (17.1) |
| COVID-19 severity upon admission | |
| Asymptomatic | 15 (2.3) |
| Mild | 78 (12.5) |
| Moderate | 206 (32.9) |
| Severe | 269 (42.8) |
| Critical | 59 (9.5) |
| Hospitalization outcome | |
| Hospitalization duration (days), mean ± SD | 13.13 ± 8.54 |
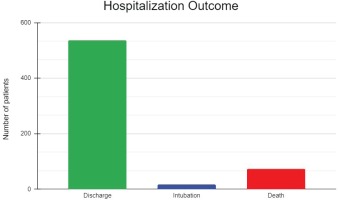
| Discharge | 537 (85.7) |
| Intubation/ICU | 17 (2.7) |
| Death | 73 (11.6)* |
Comorbidities
A total of 409 (65.2%) subjects had at least one comorbidity. Comorbidities most commonly present were arterial hypertension (AH), dyslipidemia, obesity, and diabetes mellitus (DM) (Table 1). Drugs most frequently prescribed were statins and angiotensin II receptor antagonists/ACE inhibitors (Table 1).
Hospitalization outcome
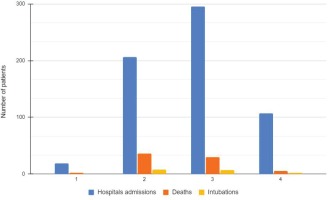
The mean (SD) duration of hospitalization was 13.13 (8.54) days, with a median of 11 days (IQR: 7.00–17.25). All 627 patients were initially admitted to the COVID-19 infectious diseases units, while 48 (7.7%) were transferred to the ICU section during their hospitalization. Finally, 537 (85.7%) were discharged, 17 (2.7%) were intubated or admitted to the ICU and subsequently discharged, and 73 (11.6%) did not survive (Figure 1, Table 2). Among those who died, 43 (6.9%) patients died in the infectious disease units and 31 (4.9%) in the ICU. The intubation rates were 0%, 3.9%, 2.3%, and 1.8% during the 1st, 2nd, 3rd, and 4th pandemic waves in Greece. The mortality rates were 11.1%, 17.5%, 10.2% and 4.7%, respectively (Figure 2, Table 2).
Comparison between groups
Comparison between discharged, deceased, and intubated/ICU patients
In the comparative analysis, a higher proportion of patients who died was diagnosed with some comorbidity (Table 3). More specifically, the deceased patients had a higher probability to be older and present with at least one comorbidity compared with the other two groups. Likewise, a higher proportion of these were diagnosed with AH, DM COPD, cancer, CKD, immunosuppression and treated with corticosteroids (Table 3).
Table 3
Comparison between deceased, intubated/ICU, discharged patients (N=627)
| Parameters | Death (n=73) n (%) | Intubation/ICU (n=17) n (%) | Discharged (n=537) n (%) | p |
|---|---|---|---|---|
| Age (years)a,b, mean ± SD | 77.58 ± 11.51 | 63.29 ± 8.1 | 60.60 ± 16.39 | <0.001 |
| Female | 40 (54.8) | 6 (35.3) | 224 (41.7) | 0.086 |
| COVID-19 waveb | 0.020 | |||
| 1st | 2 (2.9) | 0 (0) | 17 (3.1) | |
| 2nd | 36 (49.3) | 8 (47.1) | 162 (30.3) | |
| 3rd | 30 (42.0) | 7 (41.1) | 258 (48.0) | |
| 4th | 5 (5.8) | 2 (11.8) | 100 (18.6) | |
| Obesity | 0.097 | |||
| Yes | 14 (19.2) | 7 (41.2) | 173 (32.1) | |
| No | 59 (80.8) | 10 (58.8) | 364 (67.9) | |
| BMI, mean ± SD | 27.35 ± 6.11 | 29.52 ± 5.49 | 29.58 ± 5.37 | 0.249 |
| Age (years) >50b | <0.001 | |||
| Yes | 72 (98.6) | 16 (94.1) | 392 (73.0) | |
| No | 1 (1.4) | 1 (5.9) | 145 (27.0) | |
| Comorbidities ≥1b | <0.001 | |||
| Yes | 64 (87.7) | 13 (76.5) | 332 (58.0) | |
| No | 9 (12.3) | 4 (23.5) | 241 (42.0) | |
| DMb | 0.048 | |||
| Yes | 23 (31.5) | 2 (11.8) | 108 (20.1) | |
| No | 50 (68.5) | 15 (88.2) | 429 (79.1) | |
| Cancerb | 0.002 | |||
| Yes | 9 (12.3) | 2 (11.8) | 19 (3.5) | |
| No | 64 (87.7) | 15 (88.2) | 518 (96.5) | |
| Autoimmune disease | 0.530 | |||
| Yes | 2 (2.7) | 0 (0) | 24 (4.5) | |
| No | 71 (97.3) | 17 (100) | 513 (95.5) | |
| Cardiovascular diseaseb | <0.001 | |||
| Yes | 29 (39.7) | 4 (23.5) | 87 (16.2) | |
| No | 44 (60.3) | 13 (76.5) | 450 (83.8) | |
| Thyroid disease | 0.627 | |||
| Yes | 14 (19.2) | 7 (41.1) | 67 (12.5) | |
| No | 59 (80.8) | 10 (58.9) | 470 (87.5) | |
| Arterial hypertensionb | 0.002 | |||
| Yes | 42 (57.5) | 10 (58.9) | 227 (42.3) | |
| No | 31 (42.5) | 7 (41.1) | 310 (57.7) | |
| COPDb | 0.001 | |||
| Yes | 10 (13.7) | 0 (0) | 22 (4.1) | |
| No | 63 (86.3) | 17 (100) | 515 (95.9) | |
| Dyslipidemia | 0.428 | |||
| Yes | 30 (41.1) | 6 (35.3) | 179 (33.3) | |
| No | 43 (58.9) | 11 (64.7) | 358 (66.7) | |
| Smoking | 0.664 | |||
| Yes | 8 (11.0) | 1 (5.9) | 72 (13.5) | |
| No | 65 (89.0) | 16 (94.1) | 465 (86.5) | |
| Chronic kidney diseaseb | 0.004 | |||
| Yes | 9 (12.3) | 0 (0) | 21 (3.9) | |
| No | 64 (87.7) | 17 (100) | 516 (96.1) | |
| Immunosuppressionb | 0.042 | |||
| Yes | 9 (12.3) | 1 (5.9) | 27 (5.0) | |
| No | 64 (87.7) | 16 (94.1) | 510 (95.0) | |
| Corticosteroidsb | 0.001 | |||
| Yes | 11 (15.1) | 2 (11.8) | 25 (4.7) | |
| No | 62 (84.9) | 15 (88.2) | 512 (95.3) |
Comparison within discharged patients based on length of hospitalization
Among the 537 discharged patients the median duration of hospitalization was 11 days (IQR: 7–16). Older age and admission during the second wave were associated with a longer hospitalization (≥11 days) (Table 4). Furthermore, patients with a longer hospital stay (≥11 days) were more likely to have at least one comorbidity compared with those hospitalized <11 days (68.5 vs 49.1%, p<0.001). Similarly, arterial hypertension and diabetes mellitus were associated with longer hospitalization (Table 4).
Table 4
Comparison within discharged patients based on length of hospitalization
Predictors of hospitalization outcomes
Predictors of longer hospitalization
In the univariate regression analyses, duration of hospitalization was associated with age, arterial hypertension, diabetes, and the presence of at least one comorbidity. In the multivariate logistic regression analyses, only cardiovascular disease remained as an independent predictor of longer hospitalization (OR=1.834; 95% CI: 1.039–3.228) (Table 5).
Table 5
Predictors of longer hospitalization
Predictors of mortality
In the univariate regression analyses, male sex, advanced age, length of hospitalization, CVD, cancer, DM, arterial hypertension, COPD, CKD, the absence of obesity, and oral corticosteroids prior to admission were associated with mortality risk (Table 6). In multivariate binomial logistic regression analyses, age (HR=1.079; 95% CI: 1.045–1.115), history of malignancy (HR=1.246; 95% CI: 1.002–1.595), and COPD (HR=1.989; 95% CI: 1.025–7.999) remained as independent predictors of mortality (Table 6).
Table 6
Predictors of mortality
DISCUSSION
In the present retrospective analysis of prospectively collected data of 627 patients who were hospitalized with COVID-19 at the University Hospital of Ioannina during the period March 2020 – August 2021, the mortality rate was 11.6%. The majority of the patients in our population presented with severe COVID-19 pneumonia and respiratory failure upon admission (42.8%), while 168 (26.8%) of the patients progressed to critical disease during hospitalization. Older age, COPD and a history of malignancy were independent predictors of mortality. The median duration of hospitalization in our study was 11 days and the presence of cardiovascular disease was associated with a longer hospitalization.
Mortality among critically ill patients with COVID-19 varies significantly between published case series and ranges from 16% to 78%17-20. In an Italian case series of 96 patients admitted to Respiratory Intermediate Care Unit (RICU), the mortality was 46%, which increased further in ICU patients21. Our lower mortality rate (11.6% overall and 6.9% outside the ICU) could be attributed to the fact that our study did not refer exclusively to patients hospitalized in respiratory ICUs. This is despite the fact that many of our patients with severe or critical respiratory failure were managed in our non-ICU COVID-19 wards. These relatively good results may, at least partially, be attributed to the multidisciplinary patient management which led to the early implementation of specialist knowledge by the medical teams exchanging experiences and sharing treatment decision-making in three weekly multidisciplinary meetings. They could also be associated with the different COVID-19 variants and the different periods of the year that each study was held.
In our population with a mean age of 63 years, COPD was a comorbidity in 5.1%, in accordance with published results. For instance, in New York City the prevalence of COPD among hospitalized patients ranged from 2.4% to 14%, in Italy from 5.6 to 9.2%, and in China up to 10%22. In southern Italy, during the first COVID wave, the prevalence of respiratory diseases was 8.3%23. Among COPD patients it is known from the literature that severe physiological imbalance upon admission to ICU, severity of COPD disease and high comorbid burden are predictors of mortality in critically ill patients with acute COPD exacerbation24. The presence of COPD appears in turn to increase the mortality risk in patients with COVID-19. Our data, indicating that COPD is a significant mortality risk factor, are consistent with the results of published studies. In a cohort conducted in Wuhan (n=119; 3% with COPD), non-survivors were more likely to be diagnosed with COPD than survivors (7% vs 1%)25. In another Chinese multicenter study, 15.7% of the critically ill COVID-19 patients presented with COPD, but only 2.3% of those with mild severity disease26. Overall, evidence grows stronger for COPD as a risk factor for developing severe COVID-19 disease. Indeed, the presence of COPD tripled the risk of invasive mechanical ventilation, ICU admission, or death in 1590 patients in China (OR=2.681; p=0.002)27. Moreover, in another major cohort of 3988 COVID-19 patients, COPD was shown to be an independent risk factor for mortality17.
SARS-CoV-2 is similar in triggering pneumonia to SARS-CoV, the agent causing the 2002–2003 SARS pandemic. The cellular serine protease TMPRSS2 activates the spike protein of SARS-CoV-2, present in the virus envelope, facilitating the connection of the virus to the cell’s angiotensin converting enzyme 2 (ACE-2) receptor and afterwards follows the entrance to the cell. It has been recently shown that there is a significantly higher expression of ACE-2 in COPD patients than in the control individuals. Although it has not been proven that ACE-2 expression alone confers greater susceptibility or greater severity of the disease22, our data seem to underline once again how the presence of COPD can lead to worse COVID-19 outcomes, including mortality. Our data relates to a population enrolled during the entire period of the COVID-19 pandemic, from March 2020 to August 2021, unlike other studies covering shorter periods17,25,27; in other words, COPD seemed to maintain its role as a mortality predictor in patients with COVID-19, despite the improvement in the clinical and management knowledge during the pandemic.
Another independent comorbidity in our population that increased the mortality risk was malignancy. Although this finding is not confirmed in some cohort studies19,28, it is evident in others. In a retrospective study including 2476 patients (March to May 2020) in Massachusetts, presenting with cancer or CVD doubled the mortality risk in severe COVID-19 patients (HR=2.02; 95% CI: 1.53–2.68, p<0.001; HR=1.79; 95% CI: 1.21–2.66, p=0.004, respectively)28. Although prior cardiovascular disease was an independent mortality risk factor in the univariate analysis in our study, it was not confirmed in the multivariate analysis. This could be attributed to the fact that our study took into consideration only the pre-hospitalization characteristics without any clinical data, which may not reveal certain clinically relevant risk factors.
The importance of the presence of CVD in the clinical management of the COVID-19 hospitalized patients is further confirmed by the fact that it is the only independent predictor of a longer hospital stay. The median hospitalization of our population was 11 days. The median duration of hospitalization in other countries ranged 4–21 days outside China and 4–53 days in China26. Overall, 15.4% of the patients with a shorter hospitalization than the median were hospitalized during the 4th COVID wave. These data can be plausibly explained by the improvement in the experience of both the administrative and clinical organization. Other studies have also focused on the duration of hospitalization. A cohort including 100 patients hospitalized in the Pisana University Hospital (Pisa, Italy), between March and April 2020, associated obesity with duration of hospitalization (21 vs 13 days, for obese and non-obese patients, respectively)29.
Limitations
The main limitation of our study is the evaluation of only the baseline demographic characteristics of the patients, without considering any information on the clinical, laboratory or paraclinical parameters during the hospital stay. At the same time, a significant merit is that our analyses were conducted in a large cohort of 627 patients throughout the pandemic period with data that were prospectively collected using a meticulous recording system.
CONCLUSIONS
The present large cohort conducted in a tertiary COVID-19 referral hospital during the first four waves of the ongoing pandemic period in Greece, shed new light on the role of COPD and malignancy in the mortality risk of patients hospitalized with COVID-19. The presence of cardiovascular disease, on the other hand, appeared to be an independent risk factor for a longer duration of hospital stay among the patients who were subsequently discharged. Future studies are necessary in order to confirm our findings by acquiring information related to the clinical course, laboratory, and radiographic parameters of the patients with COVID-19 admitted to the hospital.