INTRODUCTION
A successful thoracic surgery partially depends on the effectiveness of a patient’s cough, postoperatively1. Coughing ensures both airway cleaning and protection2. Cough effectiveness on airway clearance can be assessed by measuring the Peak Cough Flow (PCF) with a handheld Peak Flow Meter (PFM)3,4. Cough with a peak expiratory flow over 160 L/min could ensure a safe extubation or decannulation of critically ill and postoperative patients5. Therefore, devices leading to cough enhancement could be a lifeline to the recovery of these patients6.
The use of Positive Expiratory Pressure (PEP) devices is considered beneficial for patients who have undergone abdominal or thoracic surgery, improving their arterial blood gasses7,8, hospitalization length, and chest pathology7. PEP devices are commonly used in physiotherapy9. Clinicians often use some alternative devices to create PEP, such as water bottles (bubble-PEP device) or gloves10,11, when the access to a classic PEP device either is not available or not easy due to limited resources or other practical reasons.
Several studies have already investigated alternative devices that create PEP during exhalation by using simple tubes either sunk in the water10,12 or not13, of various tube diameters14, with proven therapeutic effect15,16.
Blow glove (an alternative PEP device creating PEP using a latex glove connected to an endotracheal tube) and Tubing PEP (alternative PEP device consisted of an oxygen tube, cut at a length of between 40 and 80 cm) experimental studies, did not include any subject participation11,17, so further research is needed to be used by patients. Additionally, an endotracheal tube is not easy found and an oxygen tube cut at specific length has a risk of structural error.
When using a PEP bottle18, an airway pressure higher than the water seal is needed. During this effort, the generated pressures increase rapidly and some patients experience symptoms of dyspnea in contrast with the PEP mask10.
The case of exhalation into a tube of a specific shape and internal diameter such as a straw, following specific verbal instructions has not yet been investigated. This might be a safe, easily accessible, environmentally friendly and easy to use alternative PEP device, with almost zero cost. Low-cost devices could be important, particularly when access to commercially available PEP devices is limited or difficult.
The present study aims to find the proper way to use the new alternative PEP device, so exhalation pressures that are therapeutically beneficial will be generated by the user. The way that this could be investigated is by establishing a proper verbal instruction of exhalation through a drinking straw combined with the most appropriate straw diameter. The device was tested for its validity and reliability in a healthy population as well as in people who have undergone thoracic surgery (Stage I). Afterwards, the effect of using a simple drinking straw as an alternative PEP device was estimated, considering the effect in the clinical profile of people who have undergone thoracic surgery (Stage II). The safety of the drinking straw is ensured by the results from the secondary parameters of the research.
METHODS
Study design
The study was conducted in two stages. In Stage I, the drinking straw was tested for its validity and reliability as a PEP device in a healthy population (Step 1) and then in people who have undergone thoracic surgery subsequently (Step 2) using a mouthpiece-manometer-drinking straw ‘temporary’ device and following specific verbal instructions. Three straws and two exhalation instructions were assessed to ensure the validity of the drinking straw as an alternative PEP device.
In Stage II (intervention) a short protocolized physiotherapy session was added to the daily routine therapeutic program of the intervention group of people who have undergone thoracic surgery, to determine the effectiveness when using a drinking straw in patients’ clinical profile. This included exhalations through a drinking straw following the most appropriate verbal instruction for 3 sets of 10 repetitions. The control group did not undergo any kind of intervention.
Subjects received the study interventions 2–3 hours following their standard post-operative physiotherapy care. This was a randomized, crossover trial conducted in the Thoracic Surgery Clinic of ‘Attikon’ University Hospital, Athens, Greece.
Participants
Twenty-five healthy individuals and twenty-four people who underwent thoracic surgery were selected to participate in the research. Four people who underwent thoracic surgery were excluded from the study; two due to presenting dizziness in sitting position, one due to vomiting tendency before performing the study protocol, and one due to re-intubation before performing the study protocol. None of the aforementioned exclusions was related to the study. Eventually, twenty people who underwent thoracic surgery participated in the study.
All healthy subjects who participated in Step 1, were employees of the Hospital, and they were all the people recruited through advertisement, males and females aged 20–50 years, from February to April 2019. Individuals with pre-existing history of respiratory disease and body mass index (BMI) <30 kg/m2 were excluded. Sample recruitment of Step 2 and intervention was performed at the Thoracic Surgery Clinic of the Hospital, from February to March 2020, and included adult patients who had undergone thoracic surgery with a mesosternal or lateral incision. Demographic and clinical data were collected such as age, height and weight, type of surgery and pre-existing pathologies, if any. All subjects were hemodynamically stable, full-conscious, with systolic pressure below 150 mmHg and they were receiving a supplemental O2 mixture of below 50%. Patients with history of chronic respiratory diseases with severe symptoms, tendency to vomit, cardiac arrhythmia due to postoperative atrial fibrillation, as well as patients with angina, were excluded.
Sample calculation
Intervention’s stage sample size calculation was performed with the software G*Power 3.1.9.7 which indicated that for f=0.3 with α=0.05 and β=0.80 a total sample of 18 participants is required for the examination of repeated ANOVA measurements within each other interaction. The effect size used for the sample calculation was based on the effect size found in a previous study with a similar topic19. In similar studies of course10,19,20, a sample of 20 volunteers has been used, which was adopted21 in this study, in order to take into account possible drop out (± 10%).
Measurements
Stage I: Validity and reliability of the drinking straw
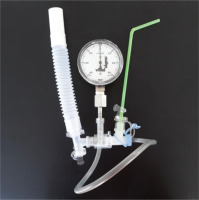
A ‘temporary’ device (Figure 1) was used to investigate the validity and reliability of the drinking straw as a PEP device, consisted of a manometer (Trademark: Vital Signs, Device accuracy: ± 2 cmH2O) to quantify the pressures developing during the exhalation attempts, a straw (of inner diameter 4, 5 or 6 mm), and a disposable mouthpiece. Before any measurement, demonstration of the proper way of using the temporary device took place. Each participant was placed in a sitting position and did not have visual contact with manometer’s indications.
Validity assessment: Step 1
The drinking straw was tested for its validity and reliability as a PEP device in healthy adults. Twenty-five healthy subjects were asked to exhale, into the ‘temporary’ device, using straws of 4, 5 and 6 mm diameter, according to one of the following exhalation instructions. Instruction A: ‘Please exhale continuously so that you feel low resistance during exhalation’; or Instruction Β: ‘Please exhale continuously so that you feel moderate resistance during exhalation’, without any further clarification. The participant was asked to exhale into the device 3 times according to one of the two instructions. This procedure was repeated for each tested straw diameter. Instruction switching was applied for each new participant to ensure randomization between instructions, whereas a rotation algorithm was used for randomization between the straws of different diameter. A rest time of 30 s was given between each straw change or each instruction switching.
The data overview of the first fourteen participants, who were asked to ‘run’ the entire program, led to the instruction that creates pressures within therapeutic limits. The remaining participants (n=11) exhaled through a drinking straw following this instruction to establish the correct straw diameter.
Validity assessment: Step 2
The drinking straw was tested for its validity and reliability as a PEP device in people who underwent thoracic surgery. Thirteen volunteers were recruited to confirm that the instruction derived from Step 1, can also be applied to people who underwent thoracic surgery.
All measurements were carried out on the 2nd and 3rd postoperative day, in the morning. The intervention protocol provided for the cessation of the trial in case a patient: experienced any difficulty, had a decrease in saturation ≤90%, felt dizzy, fainting and palpitations.
Each participant was placed in a sitting position22, with the popliteal region of the knees near the edge of the bed and stayed in this position for at least 2 minutes, to ensure that he/she would not develop any symptoms such as discoloration of the facial skin, dizziness or fainting. In case of appearance of any of the above symptoms, the protocol provided for the subject’s return back in a supine position and the immediate interruption of the procedure. Subjects who experienced very slight dizziness detected by overview or oral evaluation, remained seated until the symptom faded. In case of continuing symptom, the protocol provided for the subject’s return back in supine position and the interruption of the procedure. Participants’ respiratory rate was recorded. Saturation and heart rate were recorded using oximeter (Trademark: Nonin Onyx Vantage, Device accuracy: ± 2%) or monitor’s indications. Both systolic and diastolic blood pressure, were recorded with an analogue pressure gauge (Trademark: Fora P91, Device accuracy: ±3 mmHg). Dyspnea (BORG scale) and thoracotomy pain were quantified using the numerical pain scale (Numeric Rating Scale – oral application)23.
Subjects did not have visual contact with the manometer. The participants were asked to exhale though the ‘temporary’ device – which consisted of the most appropriate straw found in Step 1 – for 3 times as indicated by both instruction A firstly and B subsequently, or vice versa, without any further clarification. Subjects did not have visual contact with the manometer. Instruction switching was applied for each new participant to ensure randomization between the two instructions. A rest time of 30 s was given between each instruction switching. The validity of the device, both in healthy people (Step 1) and people who underwent thoracic surgery (Step 2), was estimated by comparing the average of the pressures generated by each instruction during exhalation attempts with the therapeutic range of pressures (10–20 cmH2O)24,25. Both Steps 1 and 2, demonstrated the most effective instruction, hereinafter ‘Τhe Right Instruction’.
Reliability assessment
The reliability of the straw as a PEP device was confirmed, both in healthy people and people who underwent thoracic surgery, by comparing (via the Intraclass Correlation Coefficient, ICC) the results obtained from the pressure measurements recorded by three consecutive exhalation attempts into a straw of a certain diameter by the same participant following the instructions of a pre-selected instruction.
Stage II: Intervention effectiveness
Data exported from Step 2, constituted a pilot study to determine the sample to be used at the intervention stage. A supplementary number of seven patients were employed to draw a safe conclusion. Twenty patients (13+7) were separated into two groups (10 intervention/10 control), by the sealed opaque envelopes method. Both subjects’ positioning and safety precautions were followed exactly as in Step 2. Parameters such as respiratory rate, saturation and heart rate, systolic and diastolic blood pressure and thoracotomy pain were already measured in Step 2. The intervention consisted of 3 sets of 10 exhalation repetitions10 through the most appropriate straw following the Right Instruction, including short breaks between sets. Subjects’ Peak Cough Flow (PCF) was measured using a handheld Mini Wright Peak Flow Meter (PFM) (device accuracy: ± 6.4 L/min), both before and after the end of the whole procedure. Participants applied their lips to a disposable mouthpiece connected to the PFM and were instructed to cough forcefully inside the device. The recorded value reflected the airflow during coughing and corresponded to the PCF19. Subjects supported their surgical trauma by holding a pillow on the incision during coughing19 and did not have visual contact with the PFM. The control group’s PCFs were measured following the same interval time between the measurements as the intervention group. Control group did not undergo any kind of intervention. At the end of the intervention stage, all measurements (dyspnea, pain, saturation, heart and respiratory rate, systolic and diastolic blood pressure, cough peak expiratory flow) were repeated.
Statistical analysis
Statistical analysis was conducted on IBM SPSS Statistics for Windows (Version 20.0 Armonk, NY: IBM Corp). Kolmogorov-Smirnov test was performed to check the normality of the distribution. Intraclass Correlation Coefficient (ICC) was used to assess reliability of repeated exhalations through a straw of a specific diameter. Repeated measures ANOVA with Bonferroni correction were used to assess differences amongst different straw diameters. Wilcoxon signed rank test was used to assess PCF differences amongst different instructions. Statistical significance was set at p<0.05.
RESULTS
The sample’s characteristics are shown in Tables 1, 2 and 3.
Table 1
Characteristics of sample of healthy people (N=25)
Table 2
Characteristics of sample of patients (Male: 12, Female: 8)
| Characteristics | Mean ± SD Median (IQR) |
|---|---|
| Age (years) | 64.8 ± 12.2 67.0 (15.0) |
| Weight (kg) | 83.3 ± 16.1 84.0 (25.0) |
| Height (cm) | 169.0 ± 9.0 169.0 (10.0) |
| BMI (kg/m2) | 28.6 ± 4.1 28.3 (4.7) |
Table 3
Patient’s surgery type
Table 4
Values of the dependent variables (Mean ± SD)
Step 1
Healthy participants completed the study protocol following one of the two standardized instructions.
Seven healthy participants (63 exhalations) followed instruction A; mean ± SD of expiratory pressures generated was 11.8 ± 6.1 cmH2O. Another seven healthy participants (63 exhalations) followed instruction Β; the mean expiratory pressure generated was 15.3 ± 6.9 cmH2O. There was a significant difference between the expiratory pressures generated following instruction A and instruction B; mean difference was 3.42 cmH2O (p=0.004).
Eighteen healthy participants followed instruction B (11 participants were added to the 7 healthy subjects that followed instruction B, mentioned above) with 162 exhalation attempts: mean expiratory pressures generated by instruction B, using straws with inner diameter of 4, 5, and 6 mm were 21.8 ± 12.6, 19.5 ± 10.5 and 19.9 ± 11.8 cmH2O, respectively.
Repeated measures ANOVA with Bonferroni correction did not give any statistical significant difference among the different straw diameters [F(8,10)=0.762, p=0.643].
ICC for straws of inner diameter 4, 5 and 6 mm, were 0.987, 0.979 and 0.989, respectively.
Step 2
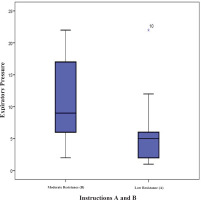
Thirteen people who underwent thoracic surgery followed both instructions A and B in a different order. The mean expiratory pressure following instruction A was 5.85 ± 5.8 cmH2O.
ICC was 0.937 (95% CI: 0.840–0.979) [F(12, 24)=15854, p<0.001] (high degree of reliability comparing expiratory pressures generated by instruction A). The mean expiratory pressure following instruction B was 10.77 ± 6.6 cmH2O. ICC was 0.889 (95% CI: 0.719–0.963) [F(12, 24)=9034, p<0.001] (high degree of reliability comparing expiratory pressures generated by instruction B).
There was a statistically significant difference between the expiratory pressures generated by the two instructions (Wilcoxon signed ranks test: Z= -2.521, p=0.012). The distribution of pressures generated by instructions A and B is shown in Figure 2.
Intervention
For intervention group (n=10), the mean of the cough expiratory flow was 169.0 ± 20.6 L/min (95% CI: 125.5–212.4) before the intervention versus 212.0 ± 21.7 L/min (95% CI: 166.3–257.6) after the intervention. For control group (n=10): the mean of the cough expiratory flow was 105.5 ± 20.6 L/min (95% CI: 62.0–148.9) before the intervention versus 119.0 ± 21.7 L/min (95% CI: 73.3–164.6) after the intervention.
The mean difference of the cough expiratory flow between the two groups before and after the intervention was 78.2 ± 28.4 L/min (95% CI: 18.4–138, p=0.013).
For 4 subjects of the intervention group, the cough expiratory flow (measured before the intervention) was below the risk limit of intubation (<160 L/min) whereas after the intervention was found to be either marginal (one subject >150 L/min) or above this limit (three subjects >160 L/min).
The mean values of the dependent variables in terms of the effect of time (before and after the intervention) and the volunteer’s group (intervention and control group) are shown in Table 3.
All secondary parameters measured before and after the intervention stage were not significantly affected by the whole procedure (Table 3).
DISCUSSION
It appears that the straw’s inner diameter does not significantly affect the results. The final selection of using a straw with 5 mm inner diameter in Step 2 and intervention stage was decided since during Step 1 the values of the pressures generated by healthy subjects exhaling into a 5 mm straw showed the smallest dispersion.
In both Step 1 (healthy subjects) and Step 2 (thoracic surgery patients), the pressures generated during exhalation following instruction A did not seem to approach the therapeutic limits of pressures. In contrast, the pressures generated during exhalation following instruction B seemed to be within the required therapeutic pressure limits, supporting the validity of the straw device. The difference between the mean values of the two instructions was statistically significant (p<0.05 in both Steps 1 and 2), which strengthens the claim that instruction B considered to be most suitable for obtaining therapeutic benefits.
In Step 2, only data corresponding to the 3rd exhalation attempt for each examined instruction was taken into account for the assessment of the validity and reliability of the straw device, since the 1st and 2nd attempt were performed in the framework of participants’ familiarity with the new device.
Repeatability of the results was found in Step 1 by examining the ICC between each participant’s exhalation attempt for each examined straw diameter following a specific instruction. Repeatability of the results was also high in Step 2, by examining the ICC between all participants’ exhalation attempts following instruction A. The same phenomenon was recorded by examining the ICC between participants’ exhalation attempts following instruction B. This probably means that since the human respiratory system can easily reproduce, with verified reliability, repeated air pressures by exhaling into a straw of specific inner diameter following a simple instruction, it could be concluded to a high degree of reliability that the straw can be used as a PEP device.
Α statistically significant increase of the PCF was recorded in all subjects of the intervention group comparing the PFM outputs before and after the intervention. Considering the PCF’s negligible increase in the control group, the use of a simple straw seems to strengthen patients’ cough.
No significant difference was recorded comparing the values of the secondary parameters of the study before and after the intervention. This supports that the use of the proposed alternative PEP device not only does not adversely affect the measured parameters, but also improves patients’ clinical overview, considering the increase of PCF.
Several attempts and many studies have been made to find alternative PEP devices in the past. In such a study, there was made an effort to create PEP using a latex glove that was connected to an endotracheal tube. In that case (blow glove), the target of pressures was obtained but without any subject participation (pressures were generated by a ventilator)11.
On the other hand, some types of alternative PEP devices, such as the tubing PEP, consist of an oxygen tube, cut at a length of 40–80 cm, depending on the patient’s needs, and therefore does not provide a fixed exhalation tube, as its length is defined either by the therapist or by the patient, and so there is a risk of structural error. Additionally, this is another case where all the above observations were made without any subject participation.
During the last few decades, the PEP bottle was introduced as another easy-to-manufacture and effective alternative device of creating PEP18 and it is now a common treatment for uncomplicated pneumonia but also used in the framework of respiratory physiotherapy. With the PEP bottle, which is a threshold resistor device, the expiratory resistance consists of a water seal. To obtain airflow within the therapeutic pressure limits, patients have to establish an airway pressure higher than the water seal, before expiration occurs. During this effort, they hold their breath and while their airways tend to overcome the above pressure threshold, the generated pressures increase rapidly and some patients experience symptoms of dyspnea. In contrast, by using a PEP mask, the airflow starts immediately at the beginning of the expiration avoiding dyspnea10. The presented alternative device, the drinking straw, follows the same logic as there is no need for patients to overcome any pressure threshold to obtain therapeutic benefits, a fact that reduces the chances of developing symptoms of dyspnea.
The Borg scale was used before and after the intervention stage to check for symptoms of dyspnea generated by using a drinking straw. The results did not show a statistically significant change, a fact that leads to the thought that a simple straw could be suitable for respiratory physiotherapy in the majority of patients.
The improvement of cough efficacy is a key priority of the treatment regimen recommended for patients who have undergone thoracic surgery22. Although the use of PEP devices as a means of respiratory physiotherapy is disputed by some researchers26, the use of a simple drinking straw resulted in a statistically significant improvement in the PCF of the intervention group, a fact which is related to their ability to manage secretions, and is a risk reduction factor for patients who have undergone thoracic surgery19.
It is also important to note that the intervention proposed in the presented study significantly contributed to the postoperative progress of some volunteers, as it helped them to increase their expiratory cough flow over the limit of 160 L/min (cough efficacy limit), which was valuable for their rehabilitation5. This is the first study to present a simple straw as an alternative PEP device. It was therefore deemed necessary to investigate not only the validity and reliability of the proposed device, but also to test it in healthy populations and patients. Hence, straws of various inner diameters were examined as well as two exhalation instructions related to low and moderate exhalation. The use of an instruction that would require violent exhalation was not assessed, to avoid triggering further pain symptoms to patients who had undergone thoracic surgery.
Limitations
Due to the COVID-19 pandemic, several preventive measures were taken by ‘Attikon’ University Hospital against the spread of the virus, among which was the closure of Thoracic Surgery Clinic.
The use of a drinking straw as an alternative PEP device needs further investigation to support the results presented in this study although a similar sample size has been used in other studies10,19,20. Patients that were participants of this research were only people who had undergone thoracic surgery. Further investigation of the drinking straw as an alternative PEP device is needed in order to be used by other types of patients.
Implications
Cough effectiveness is considered to be crucial for thoracic surgery patients’ clinical profile. Airways’ clearance and protection are ensured by having an effective cough. Positive Expiratory Pressure (PEP) devices are considered beneficial for patients who have undergone abdominal or thoracic surgery. People who have undergone thoracic surgery could perform exhalations through a simple drinking straw of 5 mm inner diameter by following the instruction ‘exhale continuously so that you feel a moderate resistance during exhalation’ to significantly increase their cough effectiveness. This suggestion needs to be further investigated in future studies.
CONCLUSIONS
This study assessed the validity, reliability and effectiveness of a drinking straw as an alternative PEP device. People who underwent thoracic surgery, using just a simple drinking straw of 5 mm inner diameter and following the instruction ‘please exhale continuously so that you feel moderate resistance during exhalation’, are able to generate pressures considered therapeutic and increase their ability to cough, which is extremely valuable for their postoperative progress. Consequently, a simple drinking straw might be an easily accessible, low cost, and efficient alternative PEP device. However, the above claims need further investigation.