INTRODUCTION
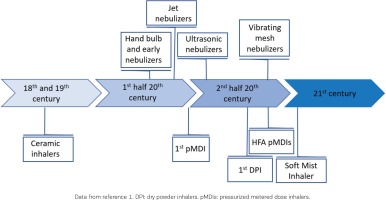
Although possibly otherwise assumed, inhalation therapy has an ancient history in the treatment of respiratory tract diseases, and, nowadays, it is well recognized as a very effective and safe method of delivering pharmaceutical compounds directly to the airways. The origins of inhaled therapies possibly go as far back as 4000 years with the smoking of datura preparations in India1. In the late 18th and 19th century, ceramic inhalers were popular for the inhalation of air drawn through infusions of plants and other compounds. A few decades later, technology evolved to atomizers, steam inhalers and powders (late 19th century), to further evolve to hand bulb nebulizers and early nebulizers using electricity or compressed air (1930s), shifting to jet or ultrasonic nebulizers, progressing to breath-enhanced, breath-actuated, vibrating mesh or dosimetric nebulizers, and finally to metered-dose liquid inhalers and soft mist inhalers (SMIs), and on the other side to pressurized MDIs (pMDIs, first introduced in 1956), breath-actuated or chlorofluorocarbon (CFC)-free MDIs, and passive or active, single- or multi-dose dry powder inhalers (DPIs) (Figure 1).
Although the inhalation route has been investigated for the systemic delivery of drugs, such as hormonal/peptide replacement, like insulin2 or analgesics, the wide use of this route of administrations involves the local treatment of pulmonary diseases, in particular asthma and chronic obstructive pulmonary disease (COPD), but also cystic fibrosis, bronchiectasis, and pulmonary arterial hypertension. The benefits of inhalation therapy include: 1) delivery of high concentrations of the active drug to the disease site; 2) minimization of systematic side effects; 3) minimization of the time between drug administration and clinical response onset; 4) bypassing important barriers that usually reduce bioavailability and therapeutic efficacy, such as poor gastrointestinal absorption and first-pass metabolism in the liver; and 5) significant reduction in the required dose to achieve an equivalent therapeutic effect compared to systematic administration3.
The type of compounds used via the inhalation route to treat pulmonary disease include mainly bronchodilators (short- or long-acting β2-adrenergic receptor agonists or muscarinic receptor antagonists, for symptomatic relief and resolution of bronchospasm, alone or in combination), corticosteroids (for ameliorating inflammatory response and preventing asthma and/or COPD exacerbations, alone or in combination with long-acting β2-adrenergic receptor agonists and long-acting muscarinic receptor antagonists), but also a variety of other agents, including antibiotics, antiviral agents, mucolytic agents, and prostacyclin. Agents that can cause bronchoconstriction (e.g. histamine or methacholine for bronchial challenge testing) may also be used, under controlled conditions for diagnostic purposes. The pulmonary pharmacokinetic processes after drug inhalation contrast to those following systemic administration, are quite complex and multi-layered. They comprise: 1) drug particle or droplet deposition (thus particle size affects therapeutic efficacy, with particles >5 μm having difficulties reaching the lower respiratory tract); 2) drug dissolution in the lung fluids; 3) mucociliary clearance in the conducting airways and macrophage clearance in the alveolar space; 4) absorption (of dissolved drug) to the lung tissue; 5) pulmonary tissue retention and potential pulmonary metabolism; and 6) absorptive drug clearance (drug transport) from the lung tissue to the systemic circulation4.
DEVELOPMENTS
Choosing the optimal device for inhalation therapy
Despite the large number of randomized controlled trials (RCTs) having been conducted on this topic, there is still no clear superiority in efficacy between the different types of devices, currently used for inhalation therapy has been demonstrated5,6. Nevertheless, some methodological limitations in the majority of these trials should be noted: first, most of these RCTs were designed for licensing purposes, thus their primary aim was to show ‘non-inferiority or at least equality’ in efficacy; second, the question of a potential superiority of one of the types of devices has not been clearly stated. Moreover, many of these RCTs have recruited patients already well trained in using the devices (i.e. when a good inhalation technique is required); while patients with poor training were excluded from the studies6. Therefore, the results of these RCTs do not present actual real-world data, i.e. they only reflect partially the population that uses inhalation devices in real life. Importantly, a systemic review by Lavorini et al.7 showed that up to 25% of patients never receive verbal instructions on how to use their devices, while another review raised the issue of evaluation of errors in technique, recording around 300 descriptions of critical errors and up to 90% of patients making technique errors, in 114 studies included8.
Advantages of MDIs include: 1) being ambulatory devices; 2) being multi-dose devices (usually contain >100 doses); 3) rapid, convenient drug delivery; 4) relatively low price; 5) ease of use; 6) content that cannot be contaminated; 7) highly reproducible dosing from puff to puff; 8) compatibility with major classes of drugs; and 9) independence from inspiratory flow generation. Disadvantages include: 1) containing propellant; 2) rarely being breath-actuated and therefore requiring coordination; 3) misuse (especially due to poor coordination), leading to ineffective therapy; 4) significant pharyngeal deposition and systematic absorption; and 5) induction of the cold freon effect. The most frequent mistakes about the use of pMDIs by the patients include: 1) forgetting to shake the device (required only in suspension formulations), 2) using the tongue or teeth as barrier into the proper diffusion of the particles, 3) inhaling too fast or too slow, 4) inhaling via the nose, 5) difficulties in synchronizing inhalation with device activation, and 6) insufficient duration of holding the breath9,10 (Table 1). In contrast, DPIs do not require propellants and do not rely on hand-inhalation coordination. Nevertheless, DPIs require a significant inhalation effort, are incompatible with many major classes of drugs, and are comparatively more complicated to use than MDIs10,11.
Table 1
pMDIs advantages and disadvantages
Another important aspect for choosing the optimal device for inhalation therapy is the capability of the targeted patients to use them. This target group consists of patients with obstructive airway disorders, mainly COPD or asthma, whose peak inspiratory flow rate (PIFR) or cognitive status might be an obstacle in effectively using some of them. DPIs require a minimal PIFR of 30–50 L/min and an optimal PIFR of 30–65 L/min, depending on the device, for adequate medication dispersal12. A significant portion of COPD patients is aged ≥70 years13,14, and age is known to be positively correlated with PIRF and cognitive decline. For instance, clinical evidence indicates that 20% of COPD patients aged >70 years using Diskus (DPI) and 30% using Turbuhaler failed to achieve the required optimal PIFR threshold for these devices (45 L/min)15. It is worth mentioning that peak pressure drops, PIFR, inhaled volume, and average inhalation flow rate do not differ markedly between healthy subjects and patients with asthma or mild COPD, but they do show a linear correlation with increased disease severity in patients with COPD16. Many studies report suboptimal or poor PIFR values for nearly half of COPD patients17,18. Similarly, asthmatic patients aged >70 years had a 6 times higher probability to fail the high resistance DPI threshold of 30 L/min PIFR compared to younger patients (aged <40 years)19. Moreover, a mini-mental examination score <24 and male gender have been shown to be an important predictor for incorrect use of pMDIs20. Finally, the cost-effectiveness of the inhalation device is also a significant factor for patients, since the annual economic burden related to the disease could reach thousands of euros21.
About 10 years ago, the European Respiratory Society and the International Society for Aerosols in Medicine agreed on a consensus recommendation to facilitate respiratory physicians in choosing the type of aerosol delivery device that is most suitable for each adult patient5. According to these guidelines, patients with a good actuation-inhalation coordination should preferably use pMDIs and those with a poor actuation-inhalation coordination should add a spacer to the pMDI. Alternatively, patients with an inspiratory flow ≥30 L/min (independent of whether they have a good or poor actuation-inhalation coordination) could use breath-actuated pMDIs or DPIs. Nebulizers are an alternative option in all patient subgroups. Spacers, despite increasing the total volume of the inhalation device as well as the therapeutic cost, are in certain cases very useful, because they simplify actuation-inhalation coordination, achieve comparatively reduced pharyngeal, and increased pulmonary, deposition of particles. pMDIs with spacers or nebulizers are also the devices of choice when conscious inhalation is not possible22 (Table 2).
Table 2
Choosing the type of aerosol delivery device
| Poor hand–inspiration coordination | Good hand–inspiration coordination | |
|---|---|---|
| Inspiratory flow <30 L/min | pMDI+spacer* | pMDI |
| Nebulizer* | Nebulizer | |
| Inspiratory flow ≥30 L/min | pMDI+spacer* | pMDI |
| Breath-actuated pMDI | Breath-actuated pMDI | |
| DPI | DPI | |
| Nebulizer* | Nebulizer |
* In case conscious inhalation is not possible. DPI: dry powder inhalers, pMDI: pressurized metered dose inhalers. Modified from data from references 5 and 22. This material has not been reviewed prior to release; therefore, the European Respiratory Society may not be responsible for any errors, omissions, or inaccuracies, or for any consequences arising there from, in the content. Reproduced with permission of the c ERS 2021. European Respiratory Journal 37 (6) 1308-1417; DOI: 10.1183/09031936.00166410 Published 1 June 2011.
Another point of concern relates to the inhalation treatment of the pediatric population with pulmonary disease, mainly asthma, where many indications are not strongly evidence-based. Age is also an important patient factor as children aged <3 years are generally unable to adopt specific inhalation techniques and are therefore treated with either nebulizers with a facemask, or pMDIs with a spacer and a facemask23. For children aged 3–6 years, pMDIs with a spacer and a facemask is the most appropriate device for use. After that age (>6 years), children are gradually more capable of using effectively DPIs and breath-activated pMDIs24. It is worth mentioning that facemasks can generally reduce the pharmacological efficacy of the inhaled drug, because: 1) they give the option of using the nasal route for drug delivery, which reduces the pulmonary deposition of the drug compared to mouth-breathing25; and 2) if not properly sealed around the nose and mouth (for example in a struggling child), pulmonary deposition of the inhaled aerosol is also reduced26. Moreover, if the child is screaming or crying, most of the inhaled drug deposits in the upper airway as well27,28.
Finally, it should be noted that, for both adults and children, the Global Initiative for Asthma (GINA)29 suggests the use of short-acting β2-adrenergic receptor agonists (SABA) in pMDI with spacer for the management of asthma exacerbations in the primary care setting, based on data supporting that delivery of SABA via hand-held devices leads to similar improvement in lung function as does a nebulizer. For children in particular, spacers may have some advantages compared to nebulizers30.
Interactive relationships between patients and inhalation devices
It is natural for medical doctors to stay alert, and continuously seek the optimal device for their patients’ inhalation therapy; a practical and cheap device, which shows a good deposition of drug particles into the lungs, the smallest possible systematic drug absorption, the capacity to effectively deliver drug particles of different sizes, and finally to match each patient’s personalized needs31. This reality potentially prompts physicians to change devices per patient until they find the best possible option. Nevertheless, various sources of evidence indicate that such a strategy is not ideal, and frequent changes between devices should be avoided to achieve good therapeutic outcomes31. For instance, a study utilizing data from the Optimum Patient Care Research Database, using a carefully matched cohort of COPD patients being prescribed the same or similar inhalation devices, and patients switching between different devices over a period of 2 years, showed that the patients in the first group had a lower rate of exacerbations and were less likely to be in a higher dose treatment group, compared with those in the different-devices cohort32.
Thus, changes in inhalation devices can increase the errors in use due to the different techniques that patients need to learn and follow. This, in turn, may lead to poor compliance to treatment, which has been correlated with unfavorable treatment outcomes and increased patient demand of primary healthcare services33-40. In contrast, simplification of treatment protocols and educating patients on the pathophysiology of the disease, interpretation of symptoms, how the drugs work, what the therapeutic goals are, and, of course, how to use inhalation devices, increase compliance and, in turn, the therapeutic outcome41. Reevaluation of technique in each visit, using the ‘teach-back’ method (asking patients to show how they use their device) either with placebo devices or by asking patients to bring their own devices to demonstrate technique, as well as participation of other healthcare professionals, such as pharmacists, in technique education are recommended by Global Initiative for Chronic Obstructive Lung Disease6. Importantly, both inhaler technique and treatment adherence must be evaluated before presuming current therapy insufficiency6.
The context, in which the therapeutic utility of a given device will be assessed, can be very complex. Before the pharmacological effect of an inhaled drug becomes a decisive factor for disease control, other parameters precede; physician–patient relationship, what is the patient’s opinion on and willingness to using medical devices, what are his/ her particular preferences in terms of the different inhalation devices, whether they are capable of using a device (factors such as age, financial issues, comorbidities or the pulmonary disease severity play here an important role), whether they will get the training required to use it properly, and finally, the performance of the device itself (correct dose, proper distribution of particles etc.). Thus, a huge variable that determines therapeutic efficacy relates to what we can call ‘physician–patient–device relationship’, which has a psychological dimension that needs to be addressed. Patients create an individualized cognitive representation of how their disease works and how this affects their life, which although being characterized by a logical structure, does not necessarily match with medical reality. This representation might be critical for patients’ willingness to use and adhere to treatment with inhalation devices42. Most patients who use inhalation devices are not keen on changing them, unless their physician strongly suggests otherwise and offers guidance/training on how the new device should be used. In case of a non-consensual switch, patients may feel unsatisfied about their treatment, insecure about the prospects of controlling their disease, downgrading the physician–patient relationship, thus reducing de facto the therapeutic efficacy and, consequently, increasing overall healthcare cost. The psychological dimension of the patient– device relationship is also nicely illustrated by the double-blind, crossover study of Wechsler et al.43, where asthmatic patients were alternating between different treatment blocks, including two blocks of inhalation device therapy, containing albuterol or placebo. Patients were reporting – subjectively – a comparable improvement in both inhalation device therapies (albuterol and placebo), even though when using objective measures for evaluating the therapeutic outcome, albuterol was the only method that significantly improved pulmonary function. The medical community seems to be aware of this psychological dimension, and up to a third of respiratory physicians seem to clearly prioritize the selection of the proper device for a given patient over the pharmacological compound, when deciding which treatment to recommend44.
The science and technology of pMDIs
pMDIs: technology
MDIs were developed about 65 years ago by Riker Laboratories and represented a convergence of two relatively new technologies at that time, the chlorofluorocarbon (CFC) propellant and the Meshburg metering valve which was originally designed for dispensing perfume45. The initial design by Riker used a glass canister coated with a vinyl plastic to improve its resilience. Since then, MDIs/pMDIs are continuously evolving to meet various technological, environmental, medical, and usability challenges.
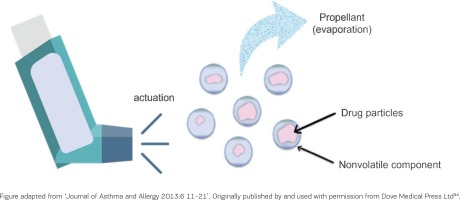
Nowadays, a typical pMDI device consists of the canister, the propellant, the drug formulation, the metering valve, and the actuator. The metering valve allows the passing of a predefined/accurately known volume of propellant and micronized drug at each actuation. The latter takes place after pressing the bottom of the canister into the actuator, leading to decompression of the formulation within the metering valve. This creates an explosive generation of a heterodispersed aerosol of droplets that consist of tiny drug particles contained within a shell of propellant. The propellant evaporates with time and distance, reducing the size of the particles under pressure to generate a metered dose of an aerosol through an atomization nozzle46,47. In 2006, pMDIs stopped using CFCs as a propellant, as they are known to damage the ozone layer (CFCs also being extremely potent global warming agents were to be replaced according to the Montreal protocol, a landmark phase-out agreement signed in 1987). Hydrofluoroalkanes (HFAs) have replaced the CFCs as propellants, as they are considered to have a lower Global Warming Potential48. HFA-based pMDIs have been introduced to allow for both: 1) suspensions, retaining same characteristics of size, deposition, and efficacy as CFCs, and 2) solutions, with varying particle size and delivery optimization49.
In this context, the Modulite® platform technology provides HFA134a-based formulations allowing modulation of aerosol clouds and adjustment of particle size, positively correlating to the concentration of the non-volatile component of the formulation (Figure 2). Over the last two decades, this technology offered notable advantages over previously existing delivery systems, including formulation adaptability, reproducibility over time and uniformity in dose delivery. The plume duration and length increased, and its velocity and contained particle size decreased compared to previous, CFC-based delivery systems50. This leads to a better peripheral lung deposition of the particles51, regardless of the presence of a pathological condition52. The extra-fine formulations achieved through the Modulite® platform technology have been linked to improved treatment efficacy in smoking asthmatic patients53, and, at a broader level, to significantly higher odds of achieving asthma control with lower exacerbation rates at significantly lower prescribed doses54.
More recent approaches for optimizing pMDI-mediated pulmonary drug delivery include Aerosphere® technology, breath-actuated pMDIs and new propellants to reduce environmental impact. In the former, porous phospholipid/ CaCl2 microparticles are used to carry drug microcrystals in a co-suspension, which irreversibly are associated with the porous particles, leading to a comparatively improved stability of the co-suspension. Moreover, this technology has a greater biocompatibility, since the phospholipids constitute a natural component of the surfactant lining in alveoli. Furthermore, the technology is characterized by higher consistency of dose (within a 15% range), irrespective of any shaking–firing delay up to one minute55,56. Altogether, these improvements lead to a more efficient lung deposition of the pharmacological compound, compared to both non extra-fine DPIs57 and suspension pMDIs58. Concerning breath-actuated pMDIs, a flow triggered mechanism is incorporated into the device and a spring releases the dose upon activation of that mechanism59. Thus, there is no need for coordination (i.e. being the major error by standard pMDI users), as low inspiratory flow is enough to trigger the mechanism60. It has been shown that patients find breath-actuated pMDIs easy to use and healthcare practitioners easy to train their patients on how to use them. Moreover, lung deposition of the pharmacological agent is comparable to good coordination, conventional pMDI usage61. Nevertheless, these devices are not available in various markets (including Greece), they are not available for a wide range of pharmacological compounds, they require good shaking and have an increased cost. Finally, new propellants for pMDIs are under investigation, such as the HFA152a, which has a low global warming potential62.
pMDIs: relationship between using technique and therapeutic efficacy
A crucial aspect of the therapeutic efficacy of pMDIs relates to the good technique that patients should have in order to properly use them. Patients usually need to uncap the mouthpiece of the device, shake the device before using it (in case of suspension containing pMDI), exhale until all air has been emptied from the lungs, place the device into their mouth, and at the very beginning of their inhalation process push down on the canister, and continue inhaling slowly and deeply. Finally, the patients are required to hold on their breath for about 5-10 seconds and then calmly exhale. Deviations from the optimal dose administration technique might affect therapeutic efficacy. For instance, fast inhalation reduces by half the percent lung deposition of drug particles >5 μm in asthmatic patients, and thus improves by less than half their FEV1 compared to slow inhalation63. These differences disappear (or even get reversed) as the size of drug particles is reduced to 3 or 1.5 μm. Another example is the importance of shaking a suspension pMDI device to provide nominal drug delivery; delayed actuation post-shaking results in varying emitted doses (32% to 380% of label dose)64. Solution pMDIs obviate the need for shaking the device.
Teaching patients how to effectively use a pMDI device is a very critical issue for the outcome of their treatment. It is concerning, though, that a great proportion of primary healthcare professionals, but also specialized pulmonologists, do not check on the adequacy of the technique of their patients in using such a device65. As a result, most patients do not realize what the critical steps are, and at least one-fifth of them aged <64 years and two-fifths of them aged ≥64 years perform one or more critical errors when using such a device66. This is a universal finding in all inhalation devices and needs a universal effort from all physicians to teach and remind the appropriate use of inhalers in all patients with airways disease at all visits and re-evaluation opportunities.
pMDIs: environmental considerations
It is an undoubtable fact that inhalation devices, such as DPIs or pMDIs can have a significant environmental impact in a type of device-dependent manner. It is also an undoubtable fact, though, that any environmental issues should be included in the wider context of designing and implementing environment friendly public policies, taking into consideration the therapeutic benefit of patients using such devices62,67.
The UK parliament has recently recommended that 50% of prescribed inhalers should have low global warming potential, and thus healthcare professionals are encouraged to switch stable patients using pMDIs to DPIs, to help achieve this goal. Moreover, the parliament recognized that, although less damaging to the environment than CFCs, HFAs still have global warming potential many times greater than CO2. Nevertheless, the impact of inhaler device choice and inhaler technique training on patient outcomes cannot be overstated, nor can we ignore the likelihood that the costs of inhaler switching to healthcare systems are potentially huge, with deteriorating asthma control leading to increased primary care consultations, prescriptions of rescue medications, emergency room visits, hospital admissions, and mortality. Thus, governments must preferably fund research in innovation hubs for alternative low global warming potential propellants and novel inhaler devices, aiming at reducing the environmental impact of inhalation devices without harming the patients68.
A recent report on determination of greenhouse gases emissions from pMDIs in Greece estimated that the pMDIs contribution represent just 0.052% of total greenhouse gases emissions. Given that SABA products comprise 50% of this market, it can be argued that a large part of these emissions comes from reliever therapy. Replacement of current propellants with HFA152-a, which has a low global warming potential, can decrease around 90% the greenhouse gases emitted by pMDIs69.
Recent suggestions have been published on how to reduce the environmental impact of inhalers in respiratory care. These include: 1) improving asthma and COPD control and reduce the use of SABA reliever inhalers, which – compared to maintenance inhalers – are considered to be the main sources of environmental burden70; 2) promoting effective self-management; 3) ensuring that all inhalers are used with correct technique for greater effectiveness; 4) making optimal use of spacers to increase clinical effectiveness of pMDIs where these are used; 5) prescribing pMDIs to minimize propellant quantity and consider alternative inhaler brand; 6) ensuring that patients have a pMDI and spacer emergency treatment pack for self-management of exacerbations, especially if using DPIs for regular treatment; 7) ensuring that pMDIs are not discarded before they are empty (value of dose counter); and 8) promoting inhaler recycling71 (Figure 3).
pMDIs in the era of telemedicine
Advances in communication and monitoring technologies will gradually be integrated into the clinical routine and the follow-up of patients, especially in the primary care setting. The COVID-19 pandemic has, indeed, already forced some relevant changes, like the significant increase of telemedicine over in-person, outpatient visits in the US and the UK72,73. In the context of the management of obstructive pulmonary disease, such technologies may be combined with the inhalation therapy to offer closer monitoring on the adherence of patients to their treatment. The latter is a very crucial determinant of treatment efficacy, as recent data highlight74. In New Zealand, the use of an inhaler with an adjusted electronic monitoring system with audiovisual reminder functions led to significant improvements in adherence to inhaled corticosteroids in school-aged children with asthma (84% vs 30% adherence in the control group)75. In Sweden, in a multicenter, physician-blinded, crossover, randomized, 8-week long trial, the implementation of a novel self-management system consisting of a patient app, a cloud-based storage solution and a healthcare interface, where patients use Bluetooth® spirometers to measure lung function, register symptoms, and receive instant feedback on asthma control and an image of the correct inhaler(s) to use and the dose, improved symptom control in patients with uncontrolled asthma compared with conventional treatment76.
Many more, different approaches have been recently tested. AIR Louisville, a collaboration forged among the Louisville Metro Government, a nonprofit institute, and a technology company, used electronic inhaler sensors to monitor where and when patients used medication. The use of this digital health platform achieved a 78% reduction in rescue inhaler use and a 48% improvement in symptom-free days. Moreover, the crowd-sourced real-world data on inhaler use, combined with environmental data, led to policy recommendations including enhancing tree canopy, tree removal mitigation, zoning for air pollution emission buffers, recommended truck routes, and developing a community asthma notification system77. In another approach, electronic medication monitors, that tracked rescue and controller inhaler medication use, were combined with a digital health platform, that presented medication use information and asthma control status to patients and providers. This intervention has been associated with lower rates of asthma-related hospitalizations and emergency room visits78. In another study, patients with severe uncontrolled (refractory) asthma were subjected to a stratified-by-site random block design, and divided into two groups, where the intensive education group received repeated training in inhaler use, adherence, and disease management, while the intervention group received the same intervention, enhanced by (bio) feedback-guided training. Repeated feedback significantly improved inhaler adherence, reducing the number of patients whose asthma – despite good adherence – remained refractory to 27% of the original number79.
Further recent examples of telemedicine using ‘smart’ inhalers include: 1) a web-based interface with integrated remote monitoring technology to deliver FeNO suppression testing to identify non-adherence to inhaled corticosteroid treatment in difficult-to-control asthmatic patients (which improved asthma control)80; 2) a cluster randomized, parallel-group, multisite, 6-month long pharmacy study on patients with obstructive pulmonary disease (asthma, COPD or combination), using an inhaler compliance assessment device attached to their maintenance inhaler, which divided patients into a biofeedback group (receiving personalized inhaler training informed by data recorded by the device), a demonstration group (receiving inhaler training and a physical demonstration with a placebo inhaler), and finally a control group (receiving usual care), that showed that community pharmacist-delivered inhaler training enhanced by a digital technology tool, improved adherence and health status of patients81; and 3) an open-label, parallel-group, 6-month, randomized controlled trial in adults with uncontrolled asthma, with the study group using a connected inhaler system, comprising clip-on inhaler sensors, a patient-facing app and a healthcare professional dashboard (showing an improved adherence, higher medication-free days and better disease control over the control group)82.
Inhalation therapy in the COVID-19 era
Since the beginning of 2020, the appearance of the novel SARS-CoV-2, causing the COVID-19 pandemic, created considerations related to the use of inhalation devices. SARS-CoV-2 is known to be transmitted via the respiratory route, through droplets or aerosols, generated – among others – during procedures (intubation, bronchoscopy) and treatments. The virus can remain viable and infectious in aerosols for hours, and on surfaces up to some days83,84. In this context, the effectiveness of non-pharmaceutical interventions (including physical distancing, case detection/ management, hygiene and safety measures) in reducing transmission rates and COVID-19 hospitalization rates/ deaths in Europe, up to April 2021, has been assessed and may be applied as response strategies to reduce the burden of COVID-19 in forthcoming waves85. Importantly, despite the lack of evidence, there is a heightened concern about the potential risk of transmission of SARS-CoV-2 in the form of aerosolized respiratory droplets during the nebulized treatment of patients with COVID-19. Therefore, the following questions have been raised: 1) should nebulized therapy be used in the hospital setting by patients infected by SARS-CoV-2; and 2) should nebulized therapy be continued in patients already using it for chronic respiratory disease management in the hospital settings.
The burden and severity of the pandemic, combined with lack of solid data on SARS-CoV-2 transmission, has pushed medical communities to provide non-specific recommendations in the form of expert opinion, such as adhering to stringent sanitation rules, the use of personal protective equipment (gloves, N95 respiratory/face masks, eye protection, gowns) in the presence of infected patients, which should be used only a single time, use of negative-pressure rooms, maintaining at least 2 m distance from patients wherever possible and the use of filters with nebulizers86. On the other hand, the Australian National Asthma Council recommends against the use of nebulizers to administer inhaled medicines, unless unavoidable, to reduce the risk of spreading the virus87. If required, GINA recommends the use of pMDI via a spacer, except for life threatening exacerbations, and adding a mouthpiece or a mask to the spacer, when needed88. Similar recommendations have been proposed by the British Thoracic Society and the British National Institute for Health and Care Excellence, despite highlighting the fact that there is no evidence supporting an increased risk of viral transmission by using nebulizers or that aerosols surrounding the nebulizer come from the nebulizer not from patients89. Finally, the Global Initiative for Chronic Obstructive Lung Disease suggests that pMDIs, DPIs and SMIs should be used for drug delivery instead of nebulizers at home, and if otherwise, the risks of nebulized therapy spreading infection to other people in patient’s homes can be minimized by avoiding use in the presence of other people and ensuring that the nebulizer is used near open windows or in areas of increased air circulation. Additionally, in cases where nebulizers may be needed in patients who are critically ill with COVID-19, receiving ventilatory support, it is vital to keep the circuit intact and prevent the transmission of the virus. Using a mesh nebulizer in patients who are ventilated allows for the addition of medication without requiring the circuit to be broken for aerosol drug delivery90. Similar guidelines have been adopted by the Greek public hospitals and medical associations and societies91,92.
Overall, it should be noted that the debate with diametrically opposed opinions, does not clarify whether it is appropriate to use nebulizers during this COVID-19 pandemic. Unfortunately, current recommendations are often in conflict with each other, and due to lack of solid evidence in producing such recommendations or even guidelines, it was not possible to follow the consolidated rigid approach that is now applied for guidance development, and it was necessary to rely on the conflicting currently available evidence and on the opinion of experts93. The sensible recommendation, however, is overall to avoid aerosol-generating procedures as much as possible, therefore the use of pMDIs with spacers could be a good alternative for most patients, especially as the pandemic continues (Figure 4).
CONCLUSION
Inhalation therapy is an ancient medicinal approach, which has evolved into providing patients and their treating physicians with a plethora of devices to manage obstructive pulmonary disease. Parameters to be carefully weighted and guide the individualized choice of an inhaler in order to achieve disease control, apart from composition, include: physician–patient relationship, patients’ opinion and willingness to use a device, their particular preferences, their capability of using a device (with inspiratory flow, coordination, duration of inhalation, age, disease severity and comorbidities being primary factors), the training required for proper use, and finally the performance of the device itself (distribution of particles and technical characteristics). The COVID-19 pandemic added even more complexity to this topic. pMDIs are one of the most frequently used types of inhalation devices for the chronic and acute management of obstructive respiratory disease. Despite having some environmental footprint and requiring a good technique by their users to achieve reliable therapeutic effects, these devices are vital tools in the arsenal of primary care physicians and pulmonologists. Switching devices for non-clinical reasons might be risky for patients with stable respiratory diseases, leading to poor disease control and increased economic burden. The ongoing research to improve the underlying technologies of pMDIs, introduce environmentally friendlier propellants and combine these devices with modern applications of telemedicine and artificial intelligence, create new pathways for the continuous utilization of these devices in the clinical routine. The future of telemedicine will be through the use of objective high-quality information that will come from commercially available devices provided directly to the patients, that will improve physician–patient communication and will save time for all parties. Smart inhalers are an integral part of telemedicine in airways disease and pMDI technology is already commercially available in certain countries.