INTRODUCTION
Although hemoptysis is a common symptom of respiratory patients, massive hemoptysis is a challenging medical emergency, usually concerning a minority of patients with extensive parenchymal diseases, such as tuberculosis and bronchiectasis1. There is growing evidence in the last years that implementing bronchoscopic hemostasis and arterial embolization in the management of massive hemoptysis has favorable outcomes in patient survival2-4.
Hemoptysis on the ground of antiplatelet agents treatment is not uncommon in clinical practice; nevertheless, such cases are usually self-limited after discontinuation of the causative agent and respond successfully to conservative treatment.
CASE PRESENTATION
A 61-year-old Caucasian male presented to the emergency department of our hospital due to hemoptysis and melena for the past 2 hours. On presentation, he was hemodynamically stable, afebrile, and hypoxemic on ambient air (PaO2: 62.5mmHg and Sat: 91%). His medical story was remarkable for coronary artery disease and dyslipidemia, whereas he had undergone percutaneous artery intervention with insertion of drug-eluting stents six months ago, due to an inferior ST-elevation myocardial infraction. Ever since, the patient had been on dual antiplatelet therapy with acetylsalicylic acid (ASA) 100 mg once daily and ticagrelor 90 mg twice daily. He did not report any bleeding episodes in the past or a family history of bleeding disorders. His hematocrit and hemoglobin levels were 41.3% and 13.9 g/dL, respectively, and his platelet (PLT) count was 90×106/dL. Coagulation studies showed activated partial thromboplastin ratio (aPTT) of 23.4 and international normalized ratio (INR) of 0.99. The patient underwent emergency gastroscopy which demonstrated blood clots in the gastric dome without evidence of active gastrointestinal bleeding. A chest computed tomography (chest CT) revealed diffuse ground glass infiltrates in the apical and posterior segments of the right upper lobe and alveolar infiltrates in the remainder of the lungs, findings compatible with diffuse alveolar hemorrhage (images could not be retrieved due to technical reasons). Recent bleeding originating from the right bronchial tree was the finding of the emergency performed bronchoscopy.
The patient was admitted to the pulmonary ward, his antiplatelet medication was discontinued, and he was initiated on ampicillin-sulbactam iv. On day 2 of hospitalization, a new episode of massive hemoptysis occurred. Despite initial conservative approach with nebulized lidocaine and adrenaline as well as fresh frozen plasma (FFP) transfusion, hemoptysis rapidly deteriorated and the patient became severely hypoxic and hemodynamically unstable. His hematocrit and hemoglobin levels rapidly dropped to 37.6% and 12.7g/dL, respectively. He was intubated and admitted to the Intensive Care Unit (ICU).
The patient was initiated on iv corticosteroids on day 2 of ICU stay as a rescue therapy since hemoptysis persisted and he was hemodynamically very unstable, despite maximum support along with FFP transfusions. On the day after corticosteroids initiation, the patient showed signs of hemodynamical stability and blood was no longer evident on bronchial inspiration. On the next day, a new bronchoscopy was performed to obtain a bronchoalveolar lavage (BAL) for further diagnostic evaluation, during which a new episode of massive bleeding originating from the right lower lobe occurred, treated with intrabronchial adrenaline infusion but BAL procedure was withheld due to temporary hemodynamical instability. Chest CT image performed 24 hours after this 2nd episode of massive hemoptysis is shown in Figure 1. The patient was eventually extubated after 6 days in the ICU and returned to the pulmonary ward, without any further hemoptysis episodes. Chest CT scan before hospital discharge is shown in Figure 2. Corticosteroids iv were continued during his hospitalization and for another 20 days after discharge in an empirical gradually reduced dosage plan. A new chest X ray and chest CT scan on day 30 after hospital discharge showed no pathological findings of the chest, with complete remission of the right upper lobe atelectasis shown on the chest CT in Figure 2.
Figure 1
Chest CT scan after the second episode of massive hemoptysis, while the patient was intubated in the ICU, showing nodular opacities and ground glass infiltrations of the right upper lobe and peribronchial opacities and infiltrations in the right lower lobe
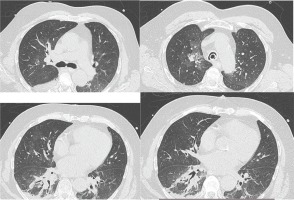
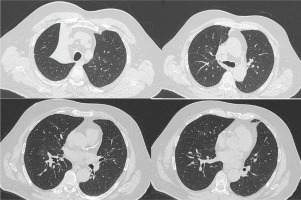
Figure 2
Chest CT scan, before hospital exit, showing right upper lobe atelectasis along with resolution of the right lung infiltrates of Figure 1. A new chest CT scan was performed 1 month after hospital discharge, in order to further investigate the possible cause of the right upper lobe atelectasis, which showed complete remission of the atelectasis and was within normal appearance
During his hospitalization, blood samples for platelet function, coagulation factors VIII and IX, von Willebrand factor and lupus anticoagulant were analyzed and were all found within normal limits. Furthermore, testing for systemic vasculitides, autoimmune diseases and connective tissue disorders (antinuclear antibodies, anti-double stranded DNA antibodies, antineutrophil cytoplasmic antibodies, perinuclear antineutrophil cytoplasmic antibodies, rheumatoid factor, complement components C3 and C4) were all within normal values. After cardiology and hematology consultations, the patient was started on aspirin 100 mg once daily and clopidogrel 75 mg once daily 5 days after ICU dismissal.
DISCUSSION
Since the chest CT was normal at 1 month examination after hospital discharge, our patient’s episodes of life-threatening massive hemoptysis were attributed to the dual antiplatelet treatment (DAPT) (ASA+ ticagrelor) for the past 6 months, either to ticagrelor alone or to its combination with ASA.
There are only limited case reports in the literature concerning pulmonary hemorrhage or diffuse alveolar hemorrhage (DAH) during ticagrelor treatment5-8. In our case, the patient was heavily bleeding in both the bronchoscopies conducted, being even hemodynamically unstable in the second one, and diagnostic approach through BAL was not feasible in either case. Thus, we could not establish the diagnosis of DAH, despite the clinical and laboratory findings (massive hemoptysis, drop of the hemoglobin level and diffuse multifocal infiltrates in chest CT) suggestive of it. Based on the large, international PLATO (Platelet Inhibition and Patient Outcomes) trial, pulmonary system was the 6th most prevalent site of major bleeding events due to ticagrelor with gastrointestinal, nose, urinary tract, skin and intracranial preceding, with the majority happening after 30 days of dual antiplatelet therapy (DAPT) with aspirin and ticagrelor9. Similarly, in the TICAKOREA trial (Ticagrelor versus Clopidogrel in Asian/Korean patients with acute coronary syndrome) pulmonary system was the 4th most prevalent location of major bleeding10, showing that pulmonary hemorrhage due to ticagrelor was quite uncommon in both trials.
Ticagrelor is an oral cyclopentryltriazolopyrimidine inhibiting the specific site of the P2Y12 platelet receptor dealing with G-protein activation and signaling11. FDA approved the use of ticagrelor for the treatment of acute coronary syndrome (ACS) in July 2011 (PLATO trial)9 and its long-term use (>1 year post ACS) in 2015 (PEGASUS-TIMI 54 trial)12. Based on the most recent 2017 ESC guidelines on ST-elevation myocardial infarction (STEMI), ticagrelor at a loading dose of 180 mg followed by 90 mg twice a day along with 75–100 mg aspirin per day is indicated for its treatment during the first year (class I recommendation, level A of evidence)13. While, based on the most recent 2020 ESC guidelines on non-ST-elevation myocardial infarction (NSTEMI), ticagrelor at a loading dose of 180 mg followed by 90 mg twice a day along with aspirin is indicated for its treatment during the first year (class I recommendation, level B of evidence)14. Other available P2Y12 inhibitors are the thienopyridines clopidogrel and prasugrel, which are administered orally as pro-drugs and they inhibit irreversibly the P2Y12 receptor11.
In contrast, ticagrelor reversibly inhibits the P2Y12 receptor and is theoretically linked to a faster action offset11. However, in our case the patient had 2 recurrent massive hemoptysis events, despite immediate discontinuation of DAPT on admission and adequate FFP transfusions during the first day of hospitalization in the pulmonary ward and then in the ICU. Moreover, clinical progress was noted only after corticosteroids were added to treatment, whereas it has been proposed that FFP may not be equally effective in cases of bleeding due to ticagrelor because of its ability to bind to the transfused platelets leading to their inhibition too11,15.
A remarkable point of interest in this case report is the time interval between initiation of DAT treatment and the onset of pulmonary hemorrhage. In previous case reports5-8, the time interval is limited to a few hours to 14 days duration of ticagrelor treatment and only Grondahl et al.16 have reported a case of a patient with DAH 10 years after initiation of dual antiplatelet therapy with ASA and prasugrel (an irreversible antagonist of P2Y12 ADP receptor). Since ticagrelor use is rising in recent years, clinicians should be able to recognize this potential adverse event even in the setting of its long-term use. Finally, PRECISE-DAPT score plays an important role in predicting bleeding events in patients with ACS undergoing percutaneous coronary intervention and subsequent dual antiplatelet therapy17.
CONCLUSION
Dual antiplatelet therapy using aspirin and ticagrelor plays a major role in the treatment of patients with ACS undergoing, or not, stent implantation as well as in patients with stable coronary artery disease and high thrombotic risk. This is also shown by the approved extended use of ticagrelor up to 3 years post ACS. A patient-tailored therapy is recommended for each patient receiving DAPT weighing up the benefits and the risks involved.
Nevertheless, pulmonary hemorrhage can occur late in the course of antiplatelet therapy. Despite its rarity, clinicians should be knowledgeable of this potentially catastrophic adverse effect to expedite diagnosis and optimize treatment. Given the fact that the majority of the existing risk scores calculating bleeding risk refers to in hospital, within 1 month and beyond 12 months after ACS, further research regarding the bleeding risk during the 1st year of DAPT should be carried out in the future. We summarize the learning points:


