INTRODUCTION
Deep vein thrombosis (DVT) and pulmonary embolism (PE) compose venous thromboembolism (VTE)1,2. PE is recorded as the third most frequent cause of cardiovascular death globally, after stroke and heart attack3. It is already recorded that most PE cases derive from DVT of the lower extremities, and almost 50% of DVT cases may cause silent PE3. Moreover, it has been demonstrated that PE is responsible for death in nearly 5% to 10% of hospitalized subjects3. Entities that could obstruct the pulmonary arteries might be tumors, clots, fat, or air, and everything that leads to this obstruction could be considered as PE3.
It is well-established that VTE and atherothrombosis may have common risk factors and pathophysiology profile, including inflammation, endothelial injury and hypercoagulability1. In addition, VTE is a clinical condition that could reinforce a pan-vascular syndrome that might consist of coronary artery disease, cerebrovascular disease, and peripheral arterial disease, while risk factors for VTE, including hypertension, diabetes mellitus (DM), cigarette smoking, and obesity, may often overlap with risk factors for atherosclerosis1,4.
Concerning PE, risk factors might be old age (>65 years), long-haul travel, associated with thrombophilia (factor V Leiden or prothrombin gene mutation), hypertension, metabolic syndrome, cigarette smoking, air pollution, obesity, postoperative, immobilization, oral contraceptives, trauma, postmenopausal, hormonal replacement, pregnancy, malignancy, and acute disease such as congestive heart failure and pneumonia1,5.
Diagnosis and clinical probability assessment of PE consist of tools such as the ‘Wells scoring system’, the D-dimer test, computed tomography pulmonary angiography (CTPA), and the VQ (ventilation perfusion) scan, are commonly utilized by clinical practitioners and other healthcare professionals to diagnose PE and VTE1,6,7.
Concerning the management of PE and treatment implementation, primary reperfusion treatment, which usually includes systemic thrombolysis, is the therapy of choice for subjects with increased risk for PE, while surgical pulmonary embolectomy or percutaneous catheter-directed therapy are other reperfusion techniques and choices in subjects who cannot sustain thrombolysis8,9. In addition, following reperfusion therapy and hemodynamic stabilization of the patient, subjects recovering from high-risk PE can be redirected from parenteral to oral anticoagulation8. In cases of intermediate-risk and low-risk PE, anticoagulant treatment is appropriate8.
Sarcopenia is a skeletal muscle mass condition that might be progressive and can be related to both skeletal muscle mass and muscle function, associated with many adverse clinical results, such as falls, disability, hospitalizations, increased hospital length of stay, and eventually higher mortality and morbidity10,11. In 2010, the European Working Group on Sarcopenia in Older People (EWGSOP) recorded an initial sarcopenia definition, while in early 2018, the Working Group (EWGSOP2) tried to update the first definition to express evidence that has been demonstrated the last years concerning this clinical condition12,13. They ended up with specific criteria that tried to depict the operational definition of sarcopenia, including: 1) low muscle strength, 2) low muscle quality or quantity, and 3) low physical performance12. Utilizing these criteria they have concluded that probable sarcopenia is validated by criterion 1, diagnosis is validated by additional documentation of criterion 2, while if criteria 1–3 are all present, sarcopenia is considered severe12.
The SARC-F questionnaire which is used to find sarcopenia cases has a low-to-moderate sensitivity and a very high specificity to forecast low muscle strength and, as a result will mainly demonstrate and record severe cases, while it can be widely used by everyday physicians12. The measurement of skeletal muscle strength might be conducted by the assessment of hand grip strength utilizing a hand grip dynamometer, while skeletal muscle quality or skeletal muscle mass might be evaluated by Dual-energy X-ray Absorptiometry (DXA), which is a commonly available method to assess muscle quantity in a non-invasive manner and Bioelectrical Impedance Analysis (BIA)12,14,15. Muscle quantity might be recorded as Appendicular Skeletal Muscle Mass (ASM), as total body Skeletal Muscle Mass (SMM), or as muscle cross-sectional area of specific muscle groups or body locations, while magnetic resonance imaging (MRI) and computed tomography (CT) are considered as gold standards for non-invasive muscle quantity/mass assessment12.
Concerning interventions that could be considered, there are no pharmacological agents for managing and treating sarcopenia16. The management of this clinical condition mainly concerns physical therapy for gait training and muscle strengthening, and training programs16. Exercise programs, especially resistance training, have been identified as promising to enhance strength and muscle mass17. In addition, specific dietary approaches and interventions, including adequate protein intake, antioxidant nutrients, vitamin D, and long-chain polyunsaturated fatty acid, have demonstrated a positive impact on sarcopenia, but more studies are needed to support these claims18. Interventions concerning exercise and combination of dietary and exercise interventions, resulted in improvement concerning decreased body muscle strength but it seems to have less consistent outcomes concerning hand grip strength and walking speed19.
In this literature review, we tried to investigate whether there is any link and interplay between the entities of sarcopenia and PE, which can both have detrimental effects and can be life-threatening medical conditions. It is already recorded that bed rest, or acute inactivity related to hospitalization or disease state, might be a potential risk to muscle tissue, muscle mass and functional capacity20,21. In addition, it is already well established that immobility is related to decreased venous blood flow, especially in the pockets of the venous valves, causing hypercoagulability, inflammation and increasing the possibility of thrombosis especially due to hospitalization or minor injuries22. As it seems, both sarcopenia and PE might be present at the abovementioned conditions, so it is imperative to study their interplay.
METHODS
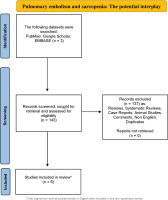
A thorough investigation was carried out among the databases of EMBASE, Google Scholar and PubMed, for the period 1976 to November 2023, utilizing the following search string: [‘sarcopenia’ OR ‘low muscle mass’ OR ‘muscle mass’] AND [‘pulmonary embolism’]. Only original studies in English were analyzed in this non-systematic review. In addition, all the references of the included studies were also exhaustively examined. Articles related to animal studies were not included in this review. The organization of the literature review is encapsulated in the flowchart diagram (Figure 1).
RESULTS
The impact of sarcopenia on PE and PE mortality
Meyer et al.23 investigated the potential role that the low skeletal muscle mass (LSMM) diagnosed by thoracic computed tomography (CT) may have for prognosis and the prediction of the mortality risk concerning cases of acute PE. They retrospectively screened the clinical database of their department for subjects who sustained acute PE between 2013 and 2017. A total of 234 patients were included in the study23. To assess the muscle mass status and the potential sarcopenia existence, they used contrast-enhanced pulmonary angiography thoracic CT to evaluate axial slides at the thoracic vertebra 5 (Th5) level23. They calculated the skeletal muscle index (SMI) by adjusting the muscle area to the subject’s height. Of their patients, 64 passed away, representing 27.4% of the initial sample23. The authors reported that SMI was slightly higher for acute PE survivors than acute PE non-survivors (57.7 ± 11.9 vs 55.6 ± 14.3 cm2/m2; p=0.07), while SMI was related to 30-day mortality in univariate, as well as multivariate analyses (respective hazard ratios, HR=1.06; 95% CI: 1.03–1.09, and HR=1.08; 95% CI: 1.04–1.11)23. This study concluded that SMI at Th5 derived from thoracic CT might have a relevant impact on 30 days mortality in subjects with acute PE and could be included in the clinical armamentarium, underlying the potential role of SMI and skeletal muscle mass health in PE23.
Akkoc et al.24 evaluated the predictive value of a certain muscle region and more specifically, of the psoas muscle area, which can be evaluated to identify sarcopenia, measured by CT concerning the forecast of in-hospital mortality in subjects with PE at admission to the intensive care unit24. A total of 89 subjects with an abdominal CT scan on admission in need of intensive care unit (ICU) confrontation were examined, while the PE severity index (PESI) was utilized to evaluate the clinical severity of the PE in accordance with the European Society of Cardiology (ESC) guidelines24. A radiologist who was blinded to subject outcomes conducted quantitative evaluation of psoas muscle anatomical regions utilizing the available CT scan depictions at the caudal end of L3 vertebra, while the psoas muscle region value was measured by dividing the sum of the right and left psoas muscle areas into the body surface area24. The rate of the in-hospital mortality was 22.5% of the subjects included in this study. It was found that the PESI of PE subjects with in-hospital mortality was significantly higher than that of the PE subjects without in-hospital mortality (p<0.05)24. In addition, the value of psoas muscle region in the PE subjects with in-hospital mortality was significantly lower than that in the PE subjects without in-hospital mortality (p<0.05)24. They concluded that the growth in the value of psoas muscle area was related to a reduction of the in-hospital mortality rate, while in subjects with in-hospital mortality associated with PE, the increased PESI and the decreased value of psoas muscle area were related to the subjects’ outcome24. This could point to the significance of psoas muscle area evaluation and sarcopenia assessment in subjects with PE, managed in intensive care units24.
In another study, Maddox et al.25 investigated the impact of sarcopenia on surgical morbidity after lower extremity (LE) reconstruction and the risk of postoperative complications25. Preoperative CT scans of the abdomen and LEs were examined to identify sarcopenia. Sarcopenia was evaluated by measuring the Hounsfield unit average calculation (HUAC) via two techniques: the ‘traditional method’ via Philips IntelliSpace Portal (ISP; Amsterdam, Netherlands) and the novel ‘ellipse method’ via CPACS25. The traditional technique was detecting the bilateral psoas at spinal level L4 with the spline contour tool and calculating bilateral psoas surface area and HU measurements. On the other hand, to conduct the novel ellipse method, area measurements and bilateral psoas density were acquired from L4, utilizing the ellipse tool instead of the spline tool25. In this study, from a total of 50 subjects given free flap-based reconstruction of the LE, 12 subjects (24%) were sarcopenic utilizing the traditional technique and 16 (32%) were sarcopenic utilizing the ellipse method, while ellipse method was shown to be more precise, sensitive, and specific compared to the traditional technique concerning the prediction of postoperative morbidity (p=0.009)25. Maddox et al.25 concluded that utilizing the ellipse technique, sarcopenic subjects were at an increased risk for any complication (p=0.002) and were at an increased hazard for a deep vein thrombus or PE utilizing the traditional technique (p=0.047)25.
Meyer et al.26 studied, utilizing thoracic CT, the pectoralis muscle region and density as a prognostic imaging biomarker of 30-day mortality in subjects with acute PE26. They retrospectively screened for subjects with thoracic CT in 3 medical centers, while pectoralis musculature was assessed on axial slices of the thoracic CT at the level of T4 of contrast enhanced pulmonary angiography CT. In addition, skeletal muscle area (SMA), SMI, muscle density and gauge were measured26. In total, 981 subjects (440 female, 44.9%) with a mean age of 63.5 ± 15.9 years were included in their study and 144 subjects (14.6%) died within the 30-days period26. Every pectoral muscle value was higher in survivors in comparison with non-survivors (for example SMI 9.9 ± 3.5 cm2/m2 vs 7.8 ± 2.6 cm2/m2, p<0.001)26. Various muscle variables are associated with 30-day mortality such as SMA (OR=0.94; 95% CI: 0.92–0.96, p<0.001); SMI (OR=0.78; 95% CI: 0.72–0.84, p<0.001); muscle density (OR=0.96; 95% CI: 0.94– 0.97, p<0.001); muscle gauge (OR=0.96; 95% CI: 0.94–0.99, p<0.001)26. Both SMI and muscle density were independently related to 30-days mortality: SMI (OR=0.81; 95% CI: 0.75–0.88, p<0.001); muscle density (OR=0.96; 95% CI: 0.95–0.98, p<0.001)26. As a result, they have concluded that parameters of the pectoralis musculature are related to 30-day mortality in subjects with acute PE26.
The impact of sarcopenia on cardiorespiratory complications including PE in subjects with colon cancer
Aro et al.27 examined whether sarcopenia or myosteatosis influence short- and long-term outcomes in subjects who were surgically managed for colorectal cancer. A total of 348 curatively treated colorectal cancer subjects were included in this retrospective study. Skeletal muscle mass and SMI were evaluated at the L3 level via venous-phase CT, while subjects were divided into sarcopenic, non-sarcopenic, and myosteatotic and non-myosteatotic27. Among their intriguing results, they demonstrated that sarcopenia was present at 208 subjects (59.8%) and myosteatosis was present at 108 subjects (31.2%). In addition, they have studied the rates of cardiorespiratory complications, among them heart failure, respiratory failure, heart attack, pleural effusion and PE, and they concluded that sarcopenic colon cancer subjects had higher cardiorespiratory complication rates compared to non-sarcopenic (6.3% vs 0.0%, p=0.023)27.
The interplay of SMI, PE and survival in subjects with esophageal cancer
Nevertheless, a study conducted by Kemper et al.28, based on the current knowledge that esophageal cancer subjects usually experience cancer-related malnutrition and consequently sarcopenia, tried to analyze the linear relation of CT-derived muscle parameters with significant clinical short- and long-term results post esophagectomy such as pneumonia, esophagoenteric leak, length of stay in ICU or hospital, pleural effusion, pleural empyema and PE, regardless of cut-offs28. In all, 98 subjects with histologically confirmed esophageal cancer were included in this study and with CT scans shortly before or after the operative procedures28. SMI, quantifying muscle mass, was evaluated by CT subjects sustaining esophagectomy, while muscle radiation attenuation (MRA) was utilized to assess muscle quality28. SMI and MRA were assessed on an axial CT slice at the height of the L3 vertebra. Logistic regression models were utilized to assess the impact of the SMI and MRA on post-surgery complications28. Concerning PE, they demonstrated that if the SMI increased by five points, the odds of a PE increased by 109.3% (95% CI: 36.6–278.3), while long-term survival analysis demonstrated that decreased MRA and decreased SMI were related to shorter survival (p=0.03)28. All the outcomes of the abovementioned studies are presented in Table 1.
Table 1
The interplay between pulmonary embolism and sarcopenia
| Authors | Study design Year | Study population | Main outcomes | Sarcopenia assessment |
|---|---|---|---|---|
| Meyer et al.23 | Retrospective 2022 | 234 subjects 64 participants passed away | SMI at Th5 from thoracic CT showed relevant impact on 30 days mortality in subjects with acute PE (HR=1.06; 95% CI: 1.03–1.09; HR=1.08; 95% CI: 1.04–1.11). SMI was slightly higher for acute PE survivors than acute PE non-survivors (57.7 ± 11.9 vs 55.6 ± 14.3 cm2/m2, p=0.07) | Axial slides at Th5 level of contrast-enhanced pulmonary angiography thoracic CT SMI calculation |
| Akkoc et al.24 | Retrospective 2020 | 89 adults In-hospital mortality rate was 22.5% | PESI of PE subjects with in-hospital mortality was significantly higher than that of the PE subjects without in-hospital mortality (p<0.05). The value of psoas muscle area in PE subjects with in-hospital mortality was significantly lower than that PE subjects without in-hospital mortality (p<0.05) | Psoas muscle area assessment using CT at the caudal end of L3 vertebra |
| Maddox et al.25 | Retrospective 2021 | A total of 50 subjects receiving free flapbased reconstruction of the LE 12 subjects (24%) sarcopenic via the traditional method and 16 (32%) sarcopenic via the ellipse method | Through ellipse method sarcopenia assessment, sarcopenic subjects were at higher risk for any complication (p=0.002) and were at a higher risk for a deep vein thrombus or PE through the traditional method of sarcopenia evaluation (p=0.047) | L4 level through tracing (‘traditional method’) and encircling (‘ellipse method’) to calculate HUAC |
| Meyer et al.26 | Retrospective 2023 | 981 subjects (440 female, 44.9%) with mean age of 63.5 ± 15.9 years 144 subjects (14.6%) died within the 30days period | Every pectoral muscle value higher in survivors compared to non-survivors (e.g. SMI 9.9 ± 3.5 cm2/m2 vs 7.8 ± 2.6 cm2/m2, p<0.001). Various muscle variables associated with 30day mortality such as SMA (OR=0.94; 95% CI: 0.92–0.96, p<0.001); SMI (OR=0.78; 95% CI: 0.72–0.84, p<0.001); muscle density (OR=0.96; 95% CI: 0.94–0.97, p<0.001); muscle gauge (OR=0.96; 95% CI: 0.94–0.99, p<0.001) Both SMI and muscle density were independently related to 30-days mortality: SMI (OR=0.81; 95% CI: 0.75– 0.88, p<0.001); muscle density (OR=0.96: 95% CI: 0.95–0.98, p<0.001) | Axial slices of the thoracic CT at the level of T4 of contrast enhanced pulmonary angiography CT |
| Aro et al.27 | Retrospective 2020 | 348 subjects curatively treated colorectal cancer | Rates of cardiorespiratory complications like heart failure, heart attack, respiratory failure, pleural effusion and PE in sarcopenic colon cancer subjects were higher than cardiorespiratory complication rates in nonsarcopenic (6.3% vs 0.0%, p=0.023) | SMI, L3 level via venous-phase CT |
| Kemper et al.28 | Retrospective 2021 | 98 subjects with histologically confirmed esophageal cancer | If SMI increased by five points, the odds of PE increased by 109.3% (95% CI: 36.6–278.3). Univariate, unadjusted long-term survival analysis demonstrated that lower MRA and lower SMI were associated with shorter survival (p=0.03) | SMI and MRA on an axial CT slice at the height of the L3 vertebra |
[i] SMI: skeletal muscle index. Th5: thoracic vertebra 5. CT: computed tomography. PE: pulmonary embolism. HR: hazard ratio. PESI: pulmonary embolism severity index. L3: third lumbar. LE: lower extremity. L4: fourth lumbar. HUAC: Hounsfield unit average calculation. SMA: skeletal muscle area. T4: fourth thoracic vertebra. MRA: muscle radiation attenuation.
DISCUSSION
This literature review article recorded the current knowledge concerning the intriguing interplay between sarcopenia and PE. These two clinical entities might share a close linkage. Sarcopenia and low muscle mass are associated with medical conditions also related to thrombotic danger such as obesity, cancer and others, resulting in both PE and sarcopenia sharing common trigger factors, even though whether there are possible underlying pathways and mechanisms concerning common pathophysiology is still an unknown matter29-34. These common factors could fuel both conditions leading to their coexistence and potentially creating a life-threatening mix with detrimental effects and poor outcomes. In addition, as already mentioned, low physical activity, prolonged bed rest, immobility and hospitalization could fuel both these conditions, leading to a potential and upcoming vicious cycle of reduced activity, disability and increased thrombosis risk20-22.
Limitations
There are some limitations that should be noted. One is that the existing literature and data that investigate this interplay are quite limited, and the studies conducted about this medical issue need to be more extensive. In addition, the number of the participants in these studies is small. Significantly, most reviewed studies are retrospective, with certain limitations that should be mentioned. Since they rely on review of charts that were not mainly planned to accumulate data for conducting research, some significant data are almost certain to be missing, and selection and recall biases also might have an impact on the outcomes and reasons for dissimilarities in therapy among subjects, while lost follow-up may often not be noted and might generally result in bias35. Moreover, in most studies, they utilized CT method to determine skeletal muscle mass or dysfunction and no other methods such as DXA which is the one most broadly utilized method in the everyday practice, as the sole radiological technique with validated cutoff values to distinguish sarcopenia36.
Implications
Studies with a greater number of participants are needed to have more validated results and conclusions. Moreover, it would be quite interesting if we could study the existence of specific biomarkers related to both sarcopenia and PE and their prognostic value in sarcopenic subjects who sustained PE while the potential and upcoming validation of prognostic indexes or scores binding these two medical issues could be quite significant. Using other radiological methods, apart from CT scan, to assess sarcopenia could be also of great importance.
An important way of managing sarcopenia is dietary interventions and the utilization of particular exercise and training strategy. On the other hand, obesity is an important hazard for PE. It would be quite remarkable if we could examine the role of specific nutritional and training interventions, which could have a protective role in both sarcopenia and PE development, or nutritional and training interventions in sarcopenic cases sustained PE to prevent recurrent events of PE.
It would be quite important everyday clinicians who treat subjects with increased risk for PE to assess these subjects further for sarcopenia and to evaluate their skeletal muscle mass utilizing existing screening and diagnostic tools, as mentioned above. Additionally, subjects who sustained PE could be evaluated for their skeletal muscle mass. This means that everyday clinical doctors should be aware of the concept of sarcopenia and skeletal muscle mass disorder and dedicate some time to sarcopenia assessment during their clinical examination of subjects having risk factors for PE. Moreover, the collaboration among physicians, dieticians and trainers could benefit subjects living with sarcopenia and having risk factors for PE or having sustained PE. A thorough follow-up of these type of patients by the abovementioned healthcare professionals could provide the optimum management with less adverse outcomes.
CONCLUSIONS
We demonstrated that sarcopenia might have an impact on subjects who sustained PE. Nevertheless, further studies should be conducted in the final analysis to obtain more answers regarding their link. Physicians and clinical doctors should be aware of this upcoming interplay and try to investigate the existence of skeletal muscle disorder and sarcopenia in subjects in danger of PE during their physical examination or in subjects who have already sustained PE, to avoid a recurrent event with potentially detrimental results.