INTRODUCTION
Patent foramen ovale (PFO) is usually a congenital heart disorder, depicting a slit-like communication between the atrial septa primum and secundum at the level of the fossa ovalis and remains oligo/asymptomatic in the majority of cases. Nevertheless, PFOs have been linked to cryptogenic strokes, paradoxical embolisms, migraine, platypnea-orthodeoxia syndrome and decompression sickness, by admixture of any venous particles to systemic arterial circulation. PFO may cause a paradoxical embolism, when right pulmonary pressures are higher than normal like in the case of relapsing pulmonary embolism or during a Valsalva maneuver, or even with normal pulmonary pressures in cases with anatomical divergences between superior vena cava and fossa ovalis1,2. Of note, even if appropriate anticoagulation therapy is applied, recurrent paradoxical embolism might occur, due to fragments of thrombus trapped in a PFO.
Contrast enhanced transthoracic or transesophageal echocardiography was established as a simple, accurate, and safe procedure for the diagnosis of interatrial communication3. Medical thrombolytic treatment or catheter thrombus fragmentation are the mainstream treatment options to restore pulmonary vascular patency and normalize right-side hemodynamics. Percutaneous PFO closure is gradually becoming the treatment of choice to restore a PFO since it prevents the recurrence of systemic thromboembolism after a first event3,4.
We present the case of a 20-year-old man with extensive thrombus in the pulmonary and left renal artery, splenic emboli and PFO, treated with anticoagulation therapy and thrombectomy, while an open-heart surgical PFO restoration is yet to follow.
CASE PRESENTATION
A 20-year-old man, active smoker, presented to our department with progressive shortness of breath gradually worsening in the last three days, and heart palpitations. He denied the presence of fever, chest pain, night sweats or weight loss. Due to a cruciate ligament injury, from a car accident twenty days ago, he remained bedridden, under prophylactic anti-coagulant treatment. The patient began physiotherapy treatment with progressive mobilization, and he stopped the anti-coagulant therapy following medical advice. He reported no medical history except for G-6-PDH deficiency and high body mass index (BMI).
Physical examination revealed a blood pressure of 180/100 mmHg, heart rate of 120 bpm, temperature 36.5°C, and oxygen saturation of 88% on ambient air. Lung auscultation was unremarkable, while abdominal examination revealed mild pain in the left hypochondriac region, gradually expanding and worsening over the following 5 hours. Cardiac auscultation revealed increased rate without murmurs or rubs, while the electrocardiography showed sinus tachycardia with S1Q3T3 pattern. There were no palpable lymph nodes. Aerial blood gases on admission revealed hypoxemia with respiratory alkalosis and lactate level of 1.2 mmol/L. Troponin levels were 950 ng/L. Renal function was within normal limits.
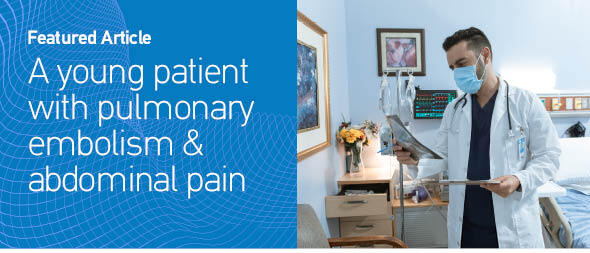
Medical history, increased levers of D-dimers, compatible clinical symptoms and the ECG findings raised the suspicion for pulmonary embolism. He was also evaluated with a bedside cardiac U/S showing increased right ventricular/left ventricular ratio and right systolic ventricular dysfunction, flattening of the intraventricular septum, with right ventricular free wall longitudinal strain of -11.4%, but no signs of intraventricular septal paradoxical motion. Consequently, a CTPA was conducted revealing filling defects of major branches of the pulmonary artery (Figure 1). The patient remained hemodynamically stable and anti-coagulant therapy with enoxaparin was immediately initiated. Two hours later, the patient complained about worsening pain in the abdominal region, not relieved with analgesics. A CTAO revealed thrombosis in the left renal artery with hypoperfusion of the kidney parenchyma and reduced blood flow in the splenic artery with concurrent infarcts (Figure 2). After a multidisciplinary-team discussion with urologists, vascular surgeons and interventional radiologists, a percutaneous suction thrombectomy was carried out successfully (Figure 3). During this procedure, part of the thrombus dislocated from the renal artery, wedged in the right common femoral artery. A surgical thrombectomy was performed, the anti-coagulant therapy with enoxaparin was replaced by unfractionated heparin with the addition of clopidogrel, after consultation of the vascular surgeons and hemostasis teams. Clopidogrel was added due to the presence of PFO and subsequent arterial thrombosis, and in view of possible underlying hypercoagulability disorder like Lupus Anticoagulant.
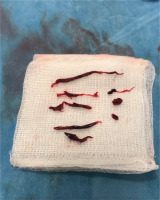
Figure 2
CTA showing thrombosis in the left renal artery with hypoperfusion of the kidney parenchyma and reduced blood flow in the splenic artery, with concurrent splenic infarcts
Phlebography of inferior limbs indicated deep vein thrombosis in the left popliteal vein. Given the presence of emboli in both the arterial and venous circulation, transesophageal echocardiography was performed. Agitated saline test during this procedure demonstrated PFO with a right-to-left atrial-level shunt and moderate pulmonary hypertension (pulmonary artery systolic pressure of 60 mmHg). A subsequent brain Magnetic Resonance Imaging (MRI) revealed a very small area with restricted diffusion in the frontotemporal part of the brain. No pathological findings were reported from the carotid and spinal artery ultrasound.
The patient was discharged with a long-term oral anticoagulation therapy, until final restoration of the PFO, having an uneventful recovery. Following multi-disciplinary team discussion, he is planned to undergo an open-heart closure of PFO, while subsequent screening for thrombophilia and other hypercoagulable disorders was negative.
DISCUSSION
Patent foramen ovale is a common anatomical variant occurring in 20–25% of the general population1. Usually, it is an incidental finding during routine cardiac investigation, or more likely remains undetected5,6. In several studies, the mortality rate associated with a paradoxical embolism secondary to a thrombus-in-transit was 18%7.
To the best of our knowledge, there have only been isolated published cases concerning patients with paradoxical emboli through a PFO leading to renal infarction8,9. Of course, only the presence of a right-to-left shunt is not enough to establish the diagnosis of paradoxical embolism. Several diagnostic criteria have been proposed, including those of Ueno et al.10 where the presence of concurrent right-to-left shunt, venous thrombosis or proven pulmonary embolism are mandatory to establish paradoxical embolism, as in our case.
Considering the high mortality rates, early diagnosis and treatment are vitally important. In that view, contrast-transesophageal echocardiography is the gold standard to detect right-to-left shunt across a PFO. An analysis of high-risk clinical factors, from the paradoxical embolism database, has shown that no correlation between the transesophageal echocardiography risk markers, such as PFO size or right-to-left shunt and other markers of cryptogenic stroke’s pathogenicity, are clearly related to paradoxical embolism11. Hypercoagulable situations such as prothrombin 20210A gene mutation have been correlated with paradoxical embolism in younger ages; yet, our patient did not present such hypercoagulable disorder12.
Secondary prophylaxis for paradoxical embolism can involve antithrombotic treatment or closure of PFO, either surgically or with a transcatheter procedure9,13. However, their efficacy is still under investigation, since large clinical trials (RESPECT - ClinicalTrials.gov number, NCT00465270, PC - ClinicalTrials.gov number, NCT00166257, and CLOSURE I - ClinicalTrials.gov number, NCT00201461) have shown no clear benefit of percutaneous PFO closure versus conventional medical therapy14. On the other hand, a review of 70 percutaneous procedures by Bissessor et el.15 has shown low rate of significant residual shunting, few impediments, and no recurrence of embolic events during a follow-up of four years. Given the small number of cases, there is no evidence that any of the treatment approaches ensures a better survival.
CONCLUSION
The presence of PFO as a cause of ischemic events in various organs, and the possibility of paradoxical embolism, should be taken into consideration when arterial embolism is suspected in cases with PE and/or DVT. This case emphasizes the presence of thrombi both in arterial and venous circulation due to PFO following PE. Transesophageal echocardiography with agitated saline test is the gold standard in the diagnosis. Immediate recognition of paradoxical embolism is needed so that anticoagulation therapy, percutaneous suction thrombectomy and percutaneous closure could restore arterial ischemia and impede more thromboembolic events. There is a pressing need for further clinical trials concerning therapeutic management.