INTRODUCTION
Nocturnal blood pressure (BP) has been reported to be a stronger predictor of cardiovascular morbidity and mortality than either daytime BP or clinic BP1,2. In addition to nocturnal BP level, nocturnal BP variability (BPV) has been found to be an independent predictor of cardiovascular events and mortality3. There is increasing evidence that fluctuations in BP levels are also associated with brain damage that may ultimately result in the development and progression of cognitive dysfunction4,5.
Obstructive sleep apnea syndrome (OSAS) induces repeated apneas and hypopneas, which causes repetitive intermittent hypoxia with consequent sympathetic activation leading to increased BP and heart rate, with repeated BP surges during sleep6,7. Thus, OSAS can lead to an increase not only in nocturnal BP level but also nocturnal BPV, each of which can independently increase cardiovascular risk8,9. Many studies have investigated hypertension (HTN)10,11, nocturnal non dipping in OSAS patients12-14. However, no study has been conducted on OSAS patients without HTN to evaluate the influence of OSA on nocturnal BP fluctuations during sleep. The purpose of this study was to evaluate nocturnal blood pressure patterns and fluctuations in OSAS patients without hypertension and determine polysomnographic parameters associated with nocturnal blood pressure fluctuations.
METHODS
This is a retrospective cross-sectional study with 100 patients of OSA diagnosed as obstructive sleep apnea based on polysomnography (PSG) full-night attended sleep monitoring (apnea hypopnea index AHI >5) and not HTN or on anti-HTN medications.
Exclusion criteria
We excluded OSAS patients diagnosed by split-night PSG monitoring or sleep monitoring of duration <3 hours. OSAS patients combined with other sleep disorders as periodic leg movement syndrome or combined with advanced pulmonary or heart diseases were also excluded from the study.
Data collection
Data were collected from patients’ medical records and polysomnographic reports. Demographic data including age, gender, BMI (kg/m2) were recorded.
Awake BP data
BP was measured using a mercury sphygmomanometer with stethoscope in the sitting position. The tourniquet was wrapped around the upper arm, keeping the arm at the same height of the heart, and a stethoscope was placed over the brachial artery. It was measured before bedtime during sleep monitoring (evening BP) and just after waking up (morning BP), and an average was recorded for awake blood pressure. A patient with BP ≥140 systolic or ≥90 diastolic was considered hypertensive14.
Polysomnography
Patients included in the study underwent full over-night attended diagnostic polysomnography (Somnomedics Somnoscreen plus device, Somnomedics, Randersacker, Germany). To record sleep stages, central and frontal EEG leads, electro-oculogram (EOG) and electromyogram of the chin (EMG) were applied. Pulse oximeter, oronasal flow thermistor and inductive plethysmography were applied for recoding oxygen variables, nasal and oral flow and thoracic and abdominal motion, respectively. Leg movements were recorded using surface electrodes placed around the middle of the tibialis anterior muscle. Other channels as ECG leads and a microphone were also applied.
Nocturnal BP monitoring
Continuous calibration of BP was started immediately after starting PSG device for all patients based on pulse transit time (PPT). PTT is defined as the time required for the arterial pulse pressure wave to travel from the aortic valve to periphery15. It can be estimated as the duration between the peak of the R wave in the ECG and the arrival of the corresponding pulse wave at the finger as determined by pulse oximetry. PTT is inversely related to BP. With increasing BP, the arterial compliance decreases and pulse wave velocity increases and thus PTT shortens16.
The patient’s BP was measured manually by a cuff-based method under resting conditions and upright sitting at start of PSG recording. The time point of this single BP measurement was entered manually at start of study. That cuff-based BP value was used for continuous calibration of the PTT based BP during the whole PSG recording using the DOMINO software.
PSG scoring
The test was scored automatically then sleep stages and respiratory events were manually revised by a sleep specialist. Those with AHI ≥5 with obstructive apneas were considered as cases of OSAS. Those with AHI ≥30 were considered severe cases of OSAS. Fifteen sleep parameters at Stage 1, Stage 2, Stage 3 and Stage REM were recorded. Parameters of respiratory events as AHI, T90 (min), ODI (oxygen desaturation index), average oxygen level, parameters of blood pressure during sleep (average systolic and diastolic BP, mean arterial pressure MAP, pulse pressure, nocturnal blood pressure fluctuation index NBPF) were recorded.
PSG definitions
Obstructive apnea was defined as the absence of airflow for at least 10 seconds in the presence of respiratory effort.
Hypopnea was considered when more than a 30% drop in airflow occurred for more than 10 seconds, associated with at least 3% oxygen desaturation or EEG arousal.
AHI is the total number of apneas and hypopneas per hour of sleep.
ODI is the number of episodes of oxygen desaturation per hour of sleep, where desaturation is defined as a drop in blood oxygen saturation to lower than 3% below baseline that lasts for at least 10 seconds.
T90 is the time spent in minutes during sleep when oxygen saturation is below 90%.
Average oxygen level is the mean oxygen saturation during sleep.
NBPF index is the increase in systolic blood pressure by more than 12 mmHg within 3–30 seconds of respiratory event per hour of sleep.
Statistical analysis
Statistical Package for the Social Sciences version 16 software (SPSS Inc.; Chicago, IL; USA) was used for analysis of results. Results are presented as mean ± standard deviation or number and percentage. The qualitative data were compared between the groups using chi-squared test. The quantitative data were compared using Student’s t-test. Spearman test was used for correlation analysis between NBPF index and respiratory sleep parameters. To predict factors associated with NBPF index, linear regression analysis was applied. A p<0.05 was considered significant.
RESULTS
The current study enrolled 100 patients with OSAS of whom 56% of patients were male. Their mean age was 56.3 ± 11.7 years. Their mean AHI was 51.5±27.4. Their PSG parameters are presented in Table 1.
Table 1
Demographic data of the study population (N=100)
According to OSAS disease severity, patients were classified into non-severe OSAS (n=56) and severe OSAS (n=44) groups. BP parameters among the two groups are presented in Table 2. Average systolic and diastolic blood pressure during sleep were significantly higher in severe OSAS group compared to non-severe OSAS group (120.1 ± 7.2 vs 114.2 ± 9.3, p<0.001; 79.6 ± 3.9 vs 73.8 ± 9.9, p<0.001, respectively). Of the nocturnal BP fluctuation parameters, NBPF index was significantly higher in severe OSAS group compared to non-severe OSAS group (41.4 ± 21.9 vs 21.6 ± 20.5, p<0.001). However, no significant difference in all awake parameters were found between the two groups (p<0.05).
Table 2
Comparison of awake and nocturnal BP parameters between non-severe OSA and severe OSA (N=100)
| Variable | Non-severe OSA (n=56) Mean ± SD | Severe OSA (n=44) Mean ± SD | p |
|---|---|---|---|
| Awake BP | |||
| Average systolic BP | 121.9 ± 8.4 | 122.3 ± 7.6 | 0.840 |
| Average diastolic BP | 79.5 ± 6.8 | 79.8 ± 4.5 | 0.799 |
| MAP | 93.6 ± 6.7 | 94.1 ± 4.7 | 0.790 |
| Pulse pressure | 41.4 ± 8.1 | 41.2 ± 6.5 | 0.970 |
| Sleep BP | |||
| Average systolic BP | 114.2 ± 9.3 | 120.1 ± 7.2 | 0.001* |
| Average diastolic BP | 73.8 ± 9.9 | 79.6 ± 3.9 | <0.001* |
| MAP | 87.3 ± 8.9 | 93.3 ± 4.2 | 0.005* |
| Pulse pressure | 41.4 ± 8.1 | 41.3 ± 6.5 | 0.931 |
| NBPF index | 21.6 ± 20.5 | 41.4 ± 21.9 | <0.001* |
| NBPF max | 26 ± 11.5 | 26.5 ± 13.8 | 0.900 |
| NBPF average | 18.5 ± 8.6 | 17.3 ± 7.7 | 0.632 |
Comparing BP parameters during sleep between rapid eye movement (REM) and non-rapid eye movement (NREM) sleep stages are presented in Table 3. Average systolic and average diastolic BP were significantly higher during REM stage compared to NREM (121.86 ± 7.7 vs 119.8 ± 7.8, p <0.001; 76.9 ± 4.6 vs 74.9 ± 4.7, p<0.001, respectively). Of the nocturnal fluctuation parameters, NBPF index as well as average NBPF were significantly higher during REM stage compared to NREM stage (37.1 ± 22.9 vs 36.7 ± 22.6, p=0.028; 17.6 ± 7.9 vs 17.1 ± 7.3, p=0.027, respectively).
Table 3
Comparison of nocturnal BP parameters between REM and NREM stages (N=100)
| Variable | REM Mean ± SD | NREM Mean ± SD | p |
|---|---|---|---|
| Sleep BP | |||
| Average systolic BP | 121.86 ± 7.7 | 119.8 ± 7.8 | <0.001* |
| Average diastolic BP | 76.9 ± 4.6 | 74.9 ± 4.7 | <0.001* |
| MAP | 91.8 ± 4.9 | 89.9 ± 5.02 | <0.001* |
| Pulse pressure | 44.9 ± 6.5 | 44.1 ± 6.6 | 0.829 |
| NBPF index | 37.1 ± 22.9 | 36.7 ± 22.6 | 0.028* |
| NBPF max | 26.2 ± 13.3 | 25.1 ± 11 | 0.051 |
| NBPF average | 17.6 ± 7.9 | 17.1 ± 7.3 | 0.027* |
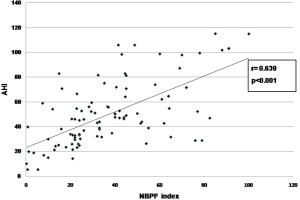
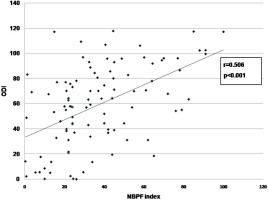
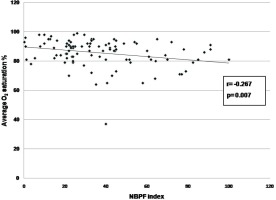
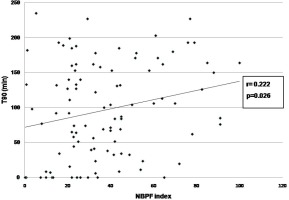
On univariate analysis, significant correlation was found between NBPF index and AHI (r=0.639, p<0.001) (Figure 1), ODI (r=0.506, p<0.001) (Figure 2), average oxygen saturation (r= -0.267, p=0.007) (Figure 3), and T90 (r=0.222, p=0.026) (Figure 4). By linear regression analysis, only AHI was a significant predictor associated with NBPF index (p<0.001) (Table 4).
Table 4
Linear regression analysis to predict nocturnal blood pressure fluctuation index in OSA patients (N=100)
| Variable | β coefficient | Standard error | p |
|---|---|---|---|
| Age (years) | 0.156 | 0.158 | 0.055 |
| BMI (kg/m2) | -0.037 | 0.094 | 0.631 |
| AHI (event/h) | 0.724 | 0.107 | <0.001* |
| Desaturation index | -0.101 | 0.100 | 0.466 |
| Average O2 saturation (%) | -0.143 | 0.246 | 0.158 |
| T90 (min) | 0.103 | 0.036 | 0.341 |
DISCUSSION
OSAS is considered a common cause of nocturnal sudden death17. Ohkubo et al18. related the mortality risk in OSAS patients to abnormal nocturnal blood pressure patterns. Many OSAS patients have non-dipping or rising BP pattern, and show BP surge in the early morning19. Nocturnal BP change is more useful to predict cardiovascular2 and cerebrovascular events4 than BP measured at clinic or home and awake BP. So the aim of this study was to evaluate nocturnal blood pressure patterns and fluctuations in OSAS patients without hypertension and determine polysomnographic parameters associated with nocturnal blood pressure fluctuations.
In this study, both average systolic and diastolic blood pressure during sleep were significantly higher in severe OSAS group compared to non-severe OSA group. NBPF index was also significantly higher in severe OSAS group compared to non-severe OSAS group as well. Previous studies have demonstrated increased arterial BP following obstructive apnea event in adult OSAS patients20-22. Furthermore, higher blood pressure levels and higher nocturnal blood pressure fluctuations were observed in severe OSAS compared to non-severe groups10,11. Several mechanisms have been proposed to explain the increase in BP during or after an obstructive respiratory event. The obstructive event during sleep is associated with increased negative intrathoracic pressure, hypoxia, hypercapnia, and arousal responses, and these changes induce increases in sympathetic activity23. During an obstructive event, the inhibitory effect of the thoracic afferents is absent, thus potentiating sympathetic activation24. As a result, vasoconstriction develops and causes a significant elevation in BP. At the end of the obstructive event, breathing resumes with abrupt elimination of vasoconstriction induced by apnea, allowing ejection of the augmented cardiac output into a peripheral vascular bed; thus, a transient increase in BP might occur in the immediate post-apnea period24,25.
Comparing sleep BP parameters among sleep stages, that study reported significant elevation of systolic BP, diastolic blood pressure during REM stage compared to NREM stage. Nocturnal blood pressure fluctuation parameters were also more during REM compared to NREM stage. Nevertheless, controversies exist in the previous data regarding the effect of sleep stages on the BP changes during obstructive respiratory events. Jelic et al.26 found that systolic BP during both REM and NREM sleep showed reduction from awake state with an increase in the post-apnea period. Garpestad et al.20 also demonstrated a higher BP elevation during post-obstructive events in both NREM and REM sleep, but the elevation was higher during REM sleep than during NREM sleep. In a population-based sample, mainly REM OSA is independently associated with non-dipping of BP in normotensive patients12.
REM induced marked hypotonia of upper airway genioglossus muscles can lead to longer apnea duration with more decrease in respiratory effort in response to upper airway occlusion during REM than NREM sleep27,28. Decrease in both hypoxic and hypercapnic ventilatory response during REM sleep compared to non-REM sleep were reported in previous studies29,30. Furthermore, apneic events were reported less frequent during slow wave sleep than REM31 and were usually associated with more hypoxemia in REM sleep32.
From the factors associated with nocturnal BP fluctuation, this study found significant correlation between NBPF index and AHI, ODI, average oxygen saturation and T90, with only AHI an independent predictor associated with NBPF index (p<0.001). Each obstructive apnea event ends with a BP peak, which lasts for few seconds and then returns to baseline33. Therefore, the higher the number of apnea events, the more surges in BP. However, the amplitude of changes in BP could be related to degree of sympathetic activity, hypoxemia at end of apnea and normotensive/hypertensive state34,35.
Controversies were reported in previous studies where some studies considered that hypoxia has the major effect on nocturnal blood pressure elevation30,36,37. Similarly, O’Donnell et al.38 in their animal study and Xu et al.39 suggest that mainly minimum oxygen saturation was associated with post-apneic BP elevation. Xu et al.40 also found that in severe OSAS, nocturnal BP levels were associated more with the nocturnal hypoxic duration and BP fluctuation than with AHI. Crinon et al.14 detected an association between OSA severity and higher nocturnal BP in a normotensive cohort where intermittent hypoxia was the most important variable related to this BP fluctuation.
However, Ringler et al.41 failed to show suppression of apnea related BP elevation despite administration of oxygen. On the other hand, Davies et al.42 reported that auditory stimulation-induced arousal in normal subjects causes large elevation in nocturnal blood pressure, which could explain acute rise in BP seen in OSAS patients following apnea events. Yoon et al.43 also reported that apnea related arousal had the strongest correlation with BP elevation during sleep in OSAS patients. Wright et al.44, by regression analysis, revealed that average 24-h MAP and mean 24-h DBP were each best predicted by change in RDI.
Limitations
Lack of hypertensive group for comparison is the main limitation of the study. Due to small sample size in our study, we could not generalize results to the general population. Further studies are recommended to evaluate nocturnal blood fluctuation in OSAS patients and effect of treatment on their outcome.
CONCLUSIONS
Nocturnal blood pressure elevations and fluctuations are common in OSAS patients without HTN, which are more in severe OSAS patients than non-severe patients, and more during REM than NREM stages. AHI is the only dependent predictor associated with nocturnal blood pressure fluctuation in OSAS patients. Thus, it is suggested that OSAS patients are at cardiovascular and cerebrovascular risk and sudden cardiac arrest during sleep. Control of apnea by CPAP might decrease cardiovascular events in those patients.