INTRODUCTION
Lung cancer is the second most prevalent cancer in both men and women in the United States, maintaining its position as the primary cause of cancer-related deaths1. Small cell lung cancer (SCLC) represents around 10–15% of the total cases. SCLC is a poorly differentiated neuroendocrine carcinoma, strongly correlated with tobacco use, which is differentiated from non-small cell lung cancer (NSCLC) by its rapid doubling time, elevated growth fraction, and the prompt onset of metastases. At the time of SCLC diagnosis, the disease is usually disseminated and treatment strategies are mostly based on systemic therapy. According to the American Veterans Administration Lung Study Group (VALG) proposal in 1957, SCLC is divided into limited and extensive stages based on whether all known tumors can be treated within a single radiotherapy field. Limited-stage SCLC (LS-SCLC) is characterized by disease confined to the ipsilateral hemithorax and regional lymph nodes, amenable to safe coverage within a radiotherapy field. Extensive-stage SCLC (ES-SCLC) denotes disease that has extended beyond these boundaries and may involve distant metastases, malignant pericardial or pleural effusions, as well as the inclusion of contralateral supraclavicular and contralateral hilar lymph node involvement. Despite the ES/LS classification’s usefulness in clinical decision-making and treatment recommendations, the AJCC 8th edition for lung cancer staging suggested the use of the TNM system which is far more accurate.
DEVELOPMENTS
Limited-Stage SCLC
According to the guidelines announced by the European Society for Medical Oncology (ESMO), the American College of Chest Physicians, and the National Comprehensive Cancer Network (NCCN), the first-line chemotherapy regimen for SCLC consists of the combination of a platinum agent (cisplatin or carboplatin) with etoposide2. The treatment efficacy of cisplatin- versus carboplatin-based chemotherapy was evaluated in the COCIS meta-analysis which suggested no differences in efficacy between the two agents for both ES and LS patients. However, carboplatin was associated with an increased frequency of high-grade hematological toxicity while cisplatin caused more severe neurological and renal toxicities as well as nausea and vomiting3.
In conjunction with chemotherapy, radiation therapy (RT) plays a significant role in the management of limited-stage small cell lung cancer (LS-SCLC). The substantial reduction in high local recurrence rates is achieved by incorporating thoracic RT. Moreover, the combination of thoracic RT with chemotherapy results in improved survival compared to chemotherapy alone, as evidenced by a comprehensive meta-analysis involving 2140 patients from 13 trials. This analysis revealed a noteworthy enhancement in the 3-year overall survival, with rates rising from 8.9% for those treated with chemotherapy alone to 14.3% for those undergoing chemoradiotherapy (hazard ratio, HR=0.86; 95% CI: 0.78–0.94; p=0.001)4.
The ideal timing for thoracic RT was examined in a phase-III trial conducted by the Japan Clinical Oncology Group (JCOG). This study compared the concurrent administration versus sequential delivery of radiotherapy in conjunction with cisplatin and etoposide for patients with limited-stage small cell lung cancer (LS-SCLC). The results showed that the median survival in the sequential arm was 19.7 months (95% CI: 15.8–23.3) and 27.2 months (95% CI: 18.4– 31.0) in the concurrent arm, thus suggesting that the concurrent chemo-radiotherapy regimen is more effective5. The standard of care for patients with LS-SCLC involves incorporating thoracic RT alongside etoposide plus cisplatin (EP) chemotherapy, initiated during either the first or second cycle. The advantages of administering thoracic RT at an early stage for individuals with LS-SCLC were reinforced by a meta-analysis involving 1524 participants from seven studies. This analysis revealed that the likelihood of survival at the two-year mark was greater for those receiving early thoracic radiation, defined as treatment initiation before the third cycle of chemotherapy, compared to those receiving late radiation (RR=1.17; 95% CI: 1.02–1.35; p=0.03)6. A randomized phase-III trial by Turrisi et al.7 compared once and twice-daily thoracic RT schedules in combination with cisplatin and etoposide in LS-SCLC patients, resulting in a significantly improved survival with the latter.
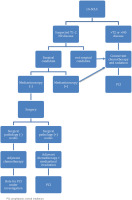
Moreover, recent data have suggested that surgical intervention may play a role in multimodality therapy for a subset of patients (5%) with early T stage, without nodal involvement (T1-T2N0M0) disease, confirmed by pathological mediastinal lymph node staging. The most effective additional treatment strategy for surgical patients has not been well defined but typically involves chemotherapy or chemoradiation. Generally, these patients exhibit a more favorable overall prognosis. An analysis of 29994 patients with clinical stages I to III small cell lung cancer (SCLC) from the National Cancer Database, revealed that among 2089 patients who underwent surgery and were matched with those who did not, those treated with surgery showed a 38-month median overall survival (OS) compared to 22 months for N0 patients. All patients with surgically resected SCLC should receive adjuvant chemotherapy with four cycles of EP, but when nodal involvement is found at the time of surgery, chemoradiation alone is recommended8 (Figure 1).
Extensive-Stage SCLC
Since the 1980s, the established treatment approach for ES-SCLC patients has involved utilizing a platinum agent in combination with etoposide. Despite various studies exploring alternative first-line chemotherapy regimens, the majority have not succeeded in modifying the established standard of care. The substitution of etoposide to irinotecan in combination with cisplatin has been evaluated in a phase-III study by the JCOG with a limited patient size (n=154), which reported encouraging results for the irinotecan-cisplatin combination with the median survival being 12.8 and 9.4 months for the irinotecan and the etoposide-containing regimens, respectively9. Nevertheless, a more extensive North American phase-III trial, with a sample size of 651, was unable to validate the previously observed advantages of irinotecan plus cisplatin (IP) as documented in Japanese patients. The median overall survival (OS) for the IP group was 9.9 months versus 9.1 months for the etoposide-cisplatin (EP) group. Intense diarrhea occurred more frequently in the case of irinotecan plus cisplatin (IP) compared to a lower incidence in the alternative regimen (19% vs 3%). Conversely, severe neutropenia and thrombocytopenia were more prevalent with etoposide plus cisplatin (EP) compared to irinotecan plus cisplatin (68% vs 33% and 15% vs 4%, respectively)10.
The great success of immune-checkpoint inhibitors (ICIs) in the treatment of non-small cell lung cancer (NSCLC) in addition to the hypothesis that SCLC is a highly immunogenic disease supported by its high mutation rate11, has led to the evaluation of ICIs’ efficacy in several clinical trials.
In 2018, the IMpower 133 trial, a substantial randomized phase-III study evaluating carboplatin plus etoposide with or without the PD-L1 immune checkpoint inhibitor atezolizumab, demonstrated an OS advantage for the group receiving immunotherapy. This double-blind, placebo-controlled phase 3 trial enrolled 403 previously untreated patients with ES-SCLC and employed a 1:1 randomization. Participants received carboplatin plus etoposide for four cycles with either atezolizumab or a placebo, followed by atezolizumab or placebo maintenance, without any requirements concerning PD-L1 expression. The median overall survival was 12.3 months in the atezolizumab group and 10.3 months in the placebo group (HR=0.70; 95% CI: 0.54–0.91; p=0.007) (Table 1). The safety characteristics of the atezolizumab group aligned with the safety profile previously documented for the individual agents. There was an equivalent occurrence of all-cause adverse events (including grade 3–4 events) between the two treatment arms. Immune-mediated AEs in the atezolizumab and the placebo group occurred in a frequency of 40% and 24%, respectively. Rash and hypothyroidism were the most common12. Exploratory subgroup analyses assessing the efficacy according to (blood-based) tumor mutational burden, were not predictive of any benefit in the atezolizumab group at either cut-off (10 or 16 mutations per megabase).
Table 1
Efficacy of immune checkpoint inhibitors in addition to chemotherapy in the first-line treatment of patients with extensive stage-small cell lung cancer as demonstrated in the three major immunotherapy phase 3 trials
CASPIAN, another randomized, phase-III trial, randomly assigned 805 treatment-naive ES-SCLC patients (1:1:1) to receive durvalumab (a PD-L1 inhibitor) plus tremelimumab (an anti-CTLA-4 antibody) plus platinum-etoposide (PE) or durvalumab plus PE or PE alone, regardless of PD-L1 expression status. Durvalumab plus tremelimumab failed to significantly improve the OS versus PE alone (HR=0.82; 95% CI: 0.68–1.00; p=0.045) with the median OS being to 10.4 months (95% CI: 9.6–12.0) versus 10.5 months (95% CI: 9.3–11.2), respectively. Durvalumab plus PE provided a continuous enhancement in overall survival versus PE alone (HR=0.75; 95% CI: 0.62–0.91; nominal p=0.0032); median overall survival was 12.9 months (95% CI: 11.3–14.7) versus 10.5 months (95% CI: 9.3–11.2) (Table 1). The most common high-grade (≥3) adverse events were neutropenia and anemia with higher frequency in the PE group (33% vs 24% in the durvalumab plus PE group)13.
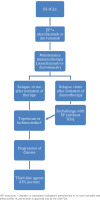
Results from the studies mentioned above have led to the approval of PD-L1 inhibitors in the first-line treatment of patients with ES-SCLC, as shown in the treatment algorithm in Figure 2.
KEYNOTE-604, a randomized, double-blind, phase-III study, evaluated the efficacy of pembrolizumab, an anti-PD-1 antibody, with etoposide and platinum (EP) versus EP with placebo, in patients with previously untreated ES-SCLC. While pembrolizumab significantly improved the progression-free survival (PFS) with 13.6% (HR=0.75; 95% CI: 0.61–0.91; p=0.0023) vs 3.1% for the EP group, it did not meet the prespecified threshold of 0.0128 for the OS (HR=0.80; 95% CI: 0.64–0.98; p=0.0164). The 24-month OS estimates were 22.5% and 11.2%, respectively, and no unexpected toxicities were observed with pembrolizumab plus EP14 (Table 1). Retrospective analyses of PD-L1 expression using the combined positive score (CPS) demonstrated similar HRs for OS and PFS in CPS>1 and CPS≤1 tumors.
In another phase-III randomized trial, Reck et al.15 evaluated the efficacy of ipilimumab, an anti-CTLA-4 antibody, plus EP versus placebo plus EP in newly diagnosed patients with ES-SCLC. The research did not yield a statistically significant enhancement in OS (HR=0.94; 95% CI: 0.81–1.09; p=0.3775), as the median OS was 11.0 months for the ipilimumab group and 10.9 months for the control group15. Maintenance therapy with nivolumab, an anti-PD-1 antibody, or nivolumab plus ipilimumab in ES-SCLC patients after 4 cycles of chemotherapy, failed to show a statistically significant enhancement in overall survival (OS). In CheckMate 451834 patients were randomly assigned (1:1:1) in three groups to receive nivolumab plus ipilimumab or nivolumab alone or placebo and the OS as 9.2 months for the nivolumab plus ipilimumab group versus 9.6 months for the placebo group (HR=0.92; 95% CI: 0.75–1.12; p=0.37). Median OS for nivolumab was 10.4 months (HR=0.84; 95% CI: 0.69–1.02)16.
Prophylactic cranial irradiation (PCI)
PCI has shown efficacy in reducing the occurrence of symptomatic brain metastases and improving overall survival in patients who have initially responded to systemic therapy. The PCI Overview Collaborative Group meta-analysis of seven randomized trials evaluating the efficacy of PCI versus no PCI in 987 patients in complete remission, demonstrated a significant reduction in the risk of developing brain disease, but also improved overall and disease-free survival. Around 85% of patients in both groups were enrolled with limited disease. The addition of PCI resulted in a 5.4% enhancement in the 3-year survival rate17. Nevertheless, findings from a Japanese randomized trial indicated that for patients without baseline brain metastases detected on MRI, PCI did not confer a survival advantage compared to a strategy involving routine surveillance MRI and subsequent treatment upon identification of asymptomatic brain metastases18.
Refractory and Relapsed SCLC
Although response rates to initial treatments are robust, responses lack durability and the majority of patients experience relapse with disease that is relatively resistant. The median survival of these patients when treated with subsequent systemic therapy is approximately 4–5 months. The time interval until disease progression influences the likelihood of response to subsequent treatment. If the disease-free interval was <3 months (resistant relapse) or there was no initial response (refractory disease), the majority of agents or treatment protocols exhibit low response rates (<10%). However, if the time to relapse was ≥3 months (sensitive relapse), anticipated response rates are higher (25%)19.
According to the NCCN guidelines, patients who relapsed more than six months after the initial treatment should be treated with the original regimen. However, patients who relapse after six months while on maintenance therapy with atezolizumab, should receive carboplatin plus etoposide (without atezolizumab)20. For patients experiencing a relapse within six months of primary therapy, the preferred regimens for use include lurbinectedin or a campothecin (most commonly topotecan) monotherapy. Numerous other agents such as irinotecan, paclitaxel, docetaxel, temozolomide, nivolumab with or without ipilimumab, pembrolizumab, vinorelbine, oral etoposide, gemcitabine, CAV (cyclophosphamide, doxorubicin and vinorelbine) and bendamustine, constitute reasonable alternatives based on phase-II trials.
Lurbinectedin is an alkylating agent approved by the FDA for use in metastatic SCLC patients on disease progression on or after a platinum-based regimen. In a single-arm, phase-II, basket trial of 105 pre-treated patients with one chemotherapy regimen, the overall response was 35.2% (95% CI: 26.2–45.2)21.
A randomized, phase-III trial compared the efficacy and safety of topotecan versus CAV in patients who had relapsed at least 60 days after completion of the initial therapy. Response rates were 24.3% for topotecan and 18.3% for the CAV group (p=0.285). Median survival was 25.0 and 24.7 weeks, respectively. Therefore, intravenous topotecan demonstrated efficacy comparable to that of the CAV regimen in the recurrent setting and it showed improved control of dyspnea, anorexia, hoarseness, and fatigue22. Another phase-III trial resulted in prolonged survival and quality of life benefit with topotecan compared with best supportive care (median survival of 25.9 vs 13.9 weeks, respectively)23. Oral compared with intravenous topotecan, in a phase-III study, demonstrated similar activity and safety and offered an alternative to IV therapy24.
Irinotecan (CPT-11) was assessed in a small phase-II trial in sixteen patients with refractory or relapsed SCLC, showing a 47% (7/15) overall response (47%; 95% CI: 21.4–71.9) with myelosuppression, diarrhea, and pulmonary toxicity being reported25.
In a phase-II study of 24 patients, paclitaxel showed a 29% (7/24) response rate (29%; 95% CI: 12–51)26. Docetaxel had similar response rates (25%; 7/28 patients) in another phase-II trial27. Temozolomide was evaluated in a phase-II trial for its safety profile showing no treatment-limiting prolonged cytopenia with the 5-day schedule, with the overall partial response being at 12% (3/25) (95% CI: 3–31). Also, temozolomide may be efficacious in patients with brain metastases, despite no noted responses in the specific trial28.
ICIs have been evaluated in several studies in patients with relapsed SCLC and could be a reasonable alternative in this setting for patients who did not receive a prior immunotherapy-containing regimen. Phase-I/II data (CheckMate 032) showed durable responses with the use of nivolumab and nivolumab plus ipilimumab in recurrent SCLC29 and led to their inclusion to the NCCN guidelines for the recurrent, platinum-refractory disease. Pembrolizumab has also been evaluated in recurrent SCLC treatment in the phase-Ib study KEYNOTE-028 and the phase-II study KEYNOTE-158. In the two studies, 83 patients were included, reporting a response rate of 19.3% (95% CI: 11.4–29.4) with the median OS being 7.7 months (95% CI: 5.2–10.1). Tumors expressing PD-L1 exhibited elevated response rates and OS30.
Sacituzumab govitecan, an antibody-drug conjugate (ADC), composed of the active metabolite of irinotecan (SN-38) linked to a humanized antibody targeting trophoblastic cell-surface antigen 2 (Trop-2), was evaluated in metastatic SCLC (mSCLC) patients in a phase I/II trial. In this study, previously pretreated mSCLC patients received 8 or 10 mg/kg of intravenous sacituzumab on days 1 and 8 of 21-day cycles. The overall response rate (ORR) was 14% and the median response duration was 5.7 months, demonstrating a safe and effective therapeutic profile31. Additional studies are required.
Another promising ADC molecule, rovalpituzumab terisine (Rova-T), has been tested in phase I and II studies. Rova-T is composed of SC16, a humanized IgG1 antibody against delta-like 3 protein (DLL3), conjugated to the cytotoxic pyrrolobenzodiazepine (PBD) by a protease-cleavable linker. In two phase I studies aiming to evaluate the safety and efficacy of Rova-T in advanced SCLC patients, findings indicated a manageable safety profile and activity supporting further exploration. However, results from the phase-II TRINITY study were not as promising as phase-I studies mentioned above. Rova-T used as 3rd line therapy and beyond in relapsed SCLC patients resulted in an ORR of 12.3% in 339 patients enrolled and 13.2% in DLL3-positive tumors by rabbit IHC32.
Recently, a phase II study evaluating the safety and efficacy of tarlatamab, a bispecific T-cell engager immunotherapy targeting delta-like ligand 3 and CD3, among individuals with previously treated SCLC, a sustained objective response rate was observed in 40% of patients, and the median overall survival reached 14.3 months. The most common adverse events included pyrexia, decreased appetite and cytokine-release syndrome33.
Molecular features
SCLC exhibits distinct genomic alterations compared to pulmonary NETs of intermediate and low grades. Nearly all SCLC patients experience loss of function alterations in the tumor suppressor genes TP53 and RB1(at 13q14). Haploinsufficiency, resulting from allele loss in various regions on chromosome 3p (including 3p21.3, 3p12, 3p14.2, and 3p24.4), leads to the absence or reduced expression of multiple tumor suppressor genes in over 90% of SCLCs, marking an early event in tumorigenesis. Genomic profiling of SCLC tumors, in addition to widespread TP53 and RB1 inactivation, reveals frequent (25%) inactivating mutations in NOTCH family genes. Mutually exclusive alterations are also common among histone acetyltransferase genes, such as CREB-binding protein (CREBBP) and E1A binding protein P300 (EP300), as well as various genes associated with TP53 and RB1. Amplification of MYC family members is detected in 20% of SCLCs. Although loss of phosphatase and tensin homolog (PTEN) is observed in 2–4% of tumors, alterations in the phosphoinositide 3-kinase (PI3K) pathway are overall more prevalent and contribute to SCLC tumorigenesis in preclinical models34.
Even with the addition of immunotherapy to frontline platinum-based chemotherapy, the enhancements in PFS and OS are relatively modest. Clinical studies involving SCLC patients have predominantly concentrated on unselected populations and have produced unsatisfactory outcomes. There is a crucial requirement for a more precise understanding of the specific characteristics of SCLC that influence its response to targeted therapies and immunotherapy. In the past few years, the categorization of SCLC subtypes has transformed from classic/variant distinctions to subsets defined by transcription factors. Gay et al.35 categorized SCLC into four subtypes primarily based on varying levels of expression of the transcription factors ASCL1, NEUROD1, and POU2F3, or by the presence of low expression in all three transcription factor signatures, along with the presence of an Inflamed gene signature. These subtypes are denoted as SCLC-A, N, P, and I, respectively35. Also, gene expression analyses in long-term survivors (LTS) of the IMpower 133 trial, identified that more LTS were treated with atezolizumab + chemotherapy than placebo + chemotherapy, and LTS in both treatment groups exhibited increased immune-related signaling. Atezolizumab and placebo showed a similar distribution of GE-defined subtypes, hinting at the possibility that the association of subtypes with treatment outcomes may have prognostic implications36.
CONCLUSION
After more than thirty years of unsuccessful clinical trials and treatment strategies in the context of SCLC, immunotherapy emerges as the most encouraging therapeutic avenue. Since immune checkpoint inhibitors and chemotherapy agents target distinct cells and pathways, combining these drugs in synergistic treatments may enhance efficacy while maintaining comparable side effects. Being still in a non-curative setting, data from the latest trials demonstrate an improvement in OS and quality of life (QoL) in SCLC patients. Moreover, the role of immunotherapy in SCLC patients with brain metastases needs to be investigated in a larger number of patients in future trials. Several trials are looking at the role of ICIs in LS-SCLC as well. Finally, recent progress in the molecular profiling of SCLC subtypes can lead the way for tailored treatment approaches.