INTRODUCTION
COPD is currently the fourth leading cause of death with approximately 3.2 million deaths in 20191,2. In the course of the disease, chronic hypercapnic respiratory failure (CHRF) can occur due to the failure of the respiratory pump3. COPD patients with CHRF have higher rates of unplanned hospital admissions and rapid clinical deterioration after hospitalization due to a severe exacerbation. Additionally, chronic hypercapnia is a decisive factor contributing to mortality4,5. Therefore, reducing factors, influencing mortality, such as exacerbation rate, is an essential therapeutic goal. Non-invasive ventilation (NIV) with a facemask is the preferred choice of treatment to reduce mortality and hospitalization rate after acute exacerbation with persistent hypercapnia6,7. However, these positive effects are evident only when NIV is used with the target of maximal possible pCO2 reduction8,9. This ventilatory strategy, based on maximal relief of the respiratory pump is referred to as high-intensity NIV therapy (HINIV). The required inspiratory positive airway pressures (IPAP) vary individually, as they depend on side effects of the NIV therapy, patient tolerance and adherence to therapy.
Despite the central therapeutic goal of reducing the exacerbation rate in the treatment of COPD patients, given its significant impact on the health condition, acceleration of lung function deterioration, worsening prognosis of the patient and responsibility for the majority of COPD-associated healthcare costs, scientific data on the effects of a HINIV therapy on the exacerbation rate are insufficient. A large German study by Koehnlein et al.7 was the first to observe reduction in mortality with the use of HINIV in COPD patients, but no conclusion could be drawn regarding the influence of HINIV on exacerbation frequency, as it was not part of the study. In a large British study investigating the effect of HINIV on hospitalization-free survival after acute exacerbation of COPD and acute respiratory failure, only severe exacerbations leading to hospitalization were recorded6. The impact of HINIV on moderate exacerbation, however, is scientifically insufficiently documented10.
The present analysis has addressed the lack of scientific evidence by initiating a study, evaluating the course of exacerbation retrospectively as well as prospectively, which compares the exacerbation frequency before initiation of HINIV and the exacerbation frequency after at least 12 months of HINIV therapy.
METHODS
The study protocol was approved by the Ethics Committee of Witten/Herdecke University and was conducted in accordance to the ethical guidelines of the declaration of Helsinki (last revised in October 2013). Written informed consent was obtained from all patients.
Patients
The data presented in this study are a preliminary analysis, which was registered at the German register for studies (DRKS00029273) and investigates supplementary telemonitoring of COPD patients after experiencing a severe exacerbation. Adult patients with the diagnosis of COPD receiving HINIV due to hypercapnic respiratory failure (PrismaVent Type 30 (n=16) and type 40 (n=4), Löwenstein medical SE & Co. KG Bad Ems, Germany) between August 2021 and September 2023, were enrolled in the study. Patients with mental retardation or those receiving invasive mechanical ventilation were not included in the study. The existing dataset of the main study was analyzed to determine availability of datasets regarding history of exacerbation as well as blood gases at the time of initiation or under existing HINIV therapy. The history of exacerbations is based on data from the clinical information system as well as from the anamnestic information provided by the patient, thus including exacerbations without hospitalization. Exacerbations that could be classified as moderate or severe according to the recommendations of the recent GOLD report were assessed10. Therefore, exacerbations requiring treatment with a fast-acting bronchodilator and oral corticosteroid (with/without antibiotics) or requiring hospitalization, were included. The anamnestic exacerbations were recorded using a predefined checklist as well as existing patient data records. The exacerbations were evaluated over the past 12 months in relation to the date of the consultation.
Ventilation setting
NIV was applied using either assisted pressure-controlled ventilation (aPCV) or pressure-supported ventilation (PSV). NIV was delivered via nasal or full-face masks. The treatment indication was based on the German guideline for treatment of chronic respiratory insufficiency, which recommends the initiation of NIV therapy in chronic hypercapnia or persistent hypercapnia after acute respiratory insufficiency3. In this case, ventilation should be performed as HINIV. HINIV refers to a specific ventilation setting in which NIV settings are aimed at achieving the lowest PaCO2 values. In HINIV, the ventilator settings are gradually increased, either up to an individually tolerated maximum value or up to the values required to achieve normocapnia8,9. If feasible, these targets should already be aimed during initiation of NIV.
Study design and measurements
Demographic data (age, sex, exacerbation history) were evaluated for all patients. Additionally, laboratory parameters (pH, pCO2, pO2, HCO3-) were documented pre-therapeutically and after a minimum of 12 months after initiation of NIV therapy. If the data were collected during acute respiratory insufficiency, the ABG values were documented immediately after admission. There was no analysis of possible consultations between these two times, as the control intervals were different for all patients and this would not allow the interim results to be compared.
Statistical analysis
The data analysis was descriptive. Group comparison was conducted using Student’s t-test for normally distributed data and the Mann-Whitney-U test for data with non-normally distributed data. Due to the small number of participants, the results are primarily intended to provide a descriptive analysis. Values of p<0.05 were therefore considered significant, although they do not provide statistical significance.
RESULTS
A total of 20 patients were included in the study (Figure 1). From those, 14 patients were female (70%). Average age of the patients was 69.2 ± 9.0 years. In the cohort analyzed, 15 patients had cardiac comorbidities, 7 of them suffering from coronary heart disease. Two patients had a sleep-related breathing disorder, one patient had chronic kidney failure, and 4 patients suffered from diabetes mellitus type II. The included patients had been treated with NIV therapy for 4.9 ± 3.5 years. Long-term NIV therapy was initiated for 9 patients, as a result of acute NIV therapy with respiratory acidosis. Patients were using the HINIV therapy 8.9 ± 4.5 h/day. The demographic data and the ventilation parameters from the time of blood gas analysis during therapy are listed in Table 1. Significant improvements in blood gas analysis were observed during therapy, compared to the results before HINV initiation. Results of the blood gas analyses are detailed in Table 2.
Table 1
Demographic data and ventilation settings during HINIV of all patients (N=20)
Table 2
Comparison of blood gas values before and after initiation of HINIV therapy of the total population (N=20)
| Pre-therapeutic Mean ± SD | HINIV Mean ± SD | p | |
|---|---|---|---|
| pH | 7.33 ± 0.1 | 7.42 ± 0.0 | <0.001 |
| pCO2 (mmHg) | 73.0 ± 22.0 | 44.0 ± 4.8 | <0.001 |
| pO2 (mmHg) | 79.0 ± 27.0 | 69.8 ± 17.3 | 0.2 |
| HCO3- (mmol/L) | 33.0 ± 4.9 | 27.9 ± 3.2 | <0.001 |
A separate analysis was conducted for patients who were initiated on NIV therapy under stable conditions without acute respiratory insufficiency. The results of this subgroup of 11 patients are presented in Table 3.
Table 3
Comparison of blood gas values before and after initiation of HINIV therapy in patients who had no acute respiratory insufficiency at the time of initiation (N=11)
| Pre-therapeutic Mean ± SD | HINIV Mean ± SD | p | |
|---|---|---|---|
| pH | 7.4 ± 0 | 7.4 ± 0 | 0.03 |
| pCO2 (mmHg) | 61.4 ± 10.6 | 45.7 ± 5.3 | <0.001 |
| pO2 (mmHg) | 84.6 ± 23.1 | 66.3 ± 15.4 | 0.08 |
| HCO3- (mmol/L) | 32.9 ± 3.6 | 28.5 ± 3.9 | 0.01 |
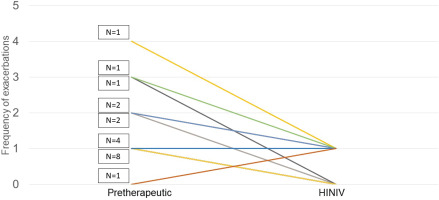
Prior to the start of HINIV therapy, patients reported an exacerbation frequency of 1.5 ± 0.9 exacerbations per year. With HINIV therapy, this frequency was successfully reduced to 0.5 ± 0.5 per year (p<0.001). In patients who had ≥2 exacerbations prior to initiation of HINIV, there was a greater mean difference in the number of exacerbations before and after therapy. Patients who had less than 2 exacerbations per year before initiation of HINIV therapy showed a mean difference in the number of exacerbations before compared to the time after initiation of therapy of 0.5 exacerbations/year versus 2 exacerbations/year in patients with ≥2 exacerbations prior HINIV. Individual differences were observed, as shown in Figure 2.
DISCUSSION
This study provides further information on reducing the frequency of moderate and severe exacerbations with established HINIV therapy. The following are the main findings, which will be discussed further.
Firstly, a significant reduction in the number of exacerbations was demonstrated with HINIV. Secondly, it has been shown that a significant reduction of hypercapnia is observed during HINIV therapy, both in the setting of acute respiratory failure and chronic respiratory failure. Thirdly, further blood gas analysis parameters showed a significant improvement under the established HINIV therapy. Fourthly, there was a strong reduction in the frequency of exacerbations, especially in patients with a high frequency of exacerbations before the HINIV therapy was initiated.
The significant reduction in exacerbation frequency under HINIV therapy differs meaningfully from previous studies that examined exacerbation frequency in hypercapnic COPD patients on NIV, but used significantly lower pressures and therefore could not reduce pCO2 (low intensity NIV)11,12. This suggests that the impact of the NIV therapy on the exacerbation frequency can only be achieved with a sufficient augmentation of ventilation and thereby a significant reduction of hypercapnia. In patients with persistent hypercapnic respiratory insufficiency, a reduction in re-admissions or mortality, following acute respiratory insufficiency, could be demonstrated after initiation of HINIV6.
Furthermore, a recently published study by Hedsund et al.13 demonstrated that the time to re-admission with an acute respiratory insufficiency, due to an exacerbation within 12 months, could be reduced by HINIV, although, a statistical significance could not be shown due to insufficient recruitment13. Nevertheless, a significant reduction in the number of exacerbations was observed, particularly in patients with frequent acute respiratory insufficiency. The lesser effects in comparison to the present study could be attributed to the fact that normocapnic patients were included as well and no re-evaluation according to current recommendations was performed14. All these studies only recorded the number of severe exacerbations that were hospitalized and did not include exacerbations that were treated in the outpatient setting.
The underlying pathophysiological mechanism of these reduced exacerbation rates resulting from HINIV therapy remains unknown. Besides the decrease in pCO2 levels, mechanical bronchial dilatation itself may alter the microbiological milieu. This has already been demonstrated with pharmacological bronchodilation15-17. It has been shown that cytokines of the airways, which are elevated in patients with COPD, can be influenced by an effective treatment with bronchodilators18. Whether these biomarkers can also be influenced by HINV therapy is the subject of ongoing research.
Limitations
The present study has several limitations that should be taken into account. The data on exacerbations were mainly based on anamnestic information from the patient. Therefore, a higher incidence of exacerbations cannot be excluded. In addition, mild exacerbations were not recorded, so it is not possible to make any statements about the effect of HINIV on these exacerbations. The data were supplemented with information from the clinical information system. In addition, the number of participants is limited, resulting in a descriptive nature of data analysis, requiring larger subsequent studies to validate the findings presented here.
CONCLUSIONS
This study shows significant positive effects of HINIV on COPD exacerbation rate, measured by frequency of moderate and severe exacerbations in patients with severe COPD. Greatest effects are observed when patients present with a high number of exacerbations prior to initiation of NIV therapy. To verify these effects in a larger cohort, further studies are needed.