INTRODUCTION
Due to the prolonged survival of patients with cystic fibrosis, we are increasingly being called upon to face new challenges in dealing with the complications and development of complications associated with the primary disease1-3. This patient developed hepatocellular carcinoma, due to cirrhosis and portal hypertension, detected on his routine annual upper abdominal ultrasound. This finding underlies the significance of annual screening tests for people with cystic fibrosis.
CASE PRESENTATION
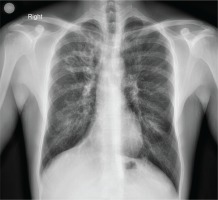
A 35-year-old patient with a history of cystic fibrosis since he was a neonate (meconium ileus) and E379X and W496X gene mutations, underwent an upper abdominal ultrasound as part of the follow-up of the known hepatic cirrhosis with concomitant portal hypertension. From the accompanying medical history, he had diabetes mellitus and was under insulin therapy, cirrhosis during adolescence, bronchiectases (Figure 1), and chronic pulmonary infection with Pseudomonas aeruginosa, while his respiratory function was as follows: Tiffeneau 60%, FVC 2.52 L (53%), FEV1 1.32 L (33%).
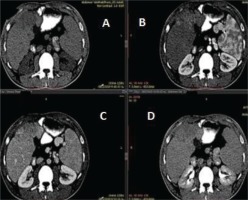
The findings of the ultrasound were consistent with the already known cirrhosis; however, heterogeneous echostructures were shown in the right lobe of the liver, which required further investigation. A three-phase computed tomography of the liver followed, which showed a space occupying lesion measuring 13×10×6 cm on the anterior surface of the right lobe, showing an uptake of the contrast agent in the arterial phase, followed by flushing of the contrast agent in the portal and delayed phase, a finding which was consistent with a hepatocellular carcinoma (Figure 2). A very high value of α-fetoprotein was initially found, at 227 ng/mL and at 665.73 ng/mL at a follow-up examination. Screening for hepatitis (B and C), metabolic, or autoimmune disorders was negative, with no history of alcoholism. Subsequently, the patient underwent magnetic resonance imaging of the abdomen, which also revealed a lesion on the anterior surface of the right lobe of the liver, with confluent nodular satellite lesions.
Figure 2
Images of a three-phase liver computed tomography. (Α) A space occupying lesion measuring 13×10×6 cm is seen on the anterior surface of the right lobe of the liver, which uptakes the contrast agent in the arterial phase (Β), followed by flushing in the portal (C) and delayed phase (D), a characteristic imaging finding that led to the diagnosis of a hepatocellular carcinoma
Based on the above imaging findings and the particularly high value of α-fetoprotein, the diagnosis of a hepatocellular carcinoma was established. Chemoembolization was not possible, due to the size of the lesion, so hepatologists suggested the initiation of an antineoplastic agent (sorafenib).
DISCUSSION
Cystic fibrosis is an inherited disease that is transmitted through its autosomal recessive nature. It is caused by a variety of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, located on chromosome 7. This gene encodes a regulatory protein (Cystic Fibrosis Transmembrane Conductance Regulator) which controls the passage of chlorine through the epithelial cell membranes of various organs of the body, such as the lungs, pancreas, liver, sweat glands, and intestine. Mutations in this gene cause reduced production or functionality of the protein, resulting in the epithelium of the affected organs producing viscous sticky mucus that obstructs the pores of the glands, resulting in progressive destruction of the organ tissue (fibrosis) and their eventual failure4,5.
With respect to hepatic involvement, mutations in the CFTR gene lead to abnormalities in composition, alkalinity, and bile flow, resulting in the production of visceral secretions in the bile ducts, which, in combination with the effect of cytotoxic or infectious agents, lead to their obstruction. Obstruction of the bile ducts subsequently leads to liver damage, resulting in the development of fibrosis, initially, and cirrhosis later on. However, the cause of the different severity of liver involvement among patients with cystic fibrosis has not been clarified. Many patients with cystic fibrosis have no clinical symptoms or signs of liver disease despite the existence of focal biliary cirrhosis4,5.
Fibrosis is thought to lead to cirrhosis over a period of years, a procedure which is usually asymptomatic2,4,5. The prevalence of liver disease in patients with CF ranges between 2% and 37%2,4. Combination of cirrhosis of the liver with portal hypertension occurs in approximately 10% of patients with CFLD (Cystic Fibrosis Liver Disease) and peaks in adolescence2 . The use of ursodeoxycholic acid improves the biochemical markers of CFLD and may slow down its development2,4. CFLD is almost always associated with pancreatic insufficiency5. One of the rare complications of liver cirrhosis in individuals with cystic fibrosis is the development of cholangiocarcinoma5. However, what is of interest in this patient case is the development of a hepatocellular carcinoma due to cirrhosis and portal hypertension, which is not related to the pathophysiology of the disease but is clearly associated to cirrhosis.
Hepatocellular carcinoma is the most common liver tumor (90–95% of primary malignant liver tumors) and the number of new cases is increasing2,6. The major causative agents are chronic hepatitis B and C infections, which account for over 80% of cases worldwide6. Other etiologic factors are alcohol and smoking, liver adenoma and repeated ingestion of aflatoxin containing foods7. The incidence ratio between men and women is 3:22.
The patient presented here developed hepatocellular carcinoma due to cirrhosis and portal hypertension, with a negative history of hepatitis B, C and alcoholism. After reviewing the literature, at least 3 more patients with CFLD have developed hepatocellular carcinoma1-3. The first case is a 32-year-old pregnant woman with cystic fibrosis and CFLD who was diagnosed with a focal liver injury during the annual upper abdominal ultrasound. After undergoing a biopsy and the diagnosis of hepatocellular carcinoma was confirmed, she was finally treated with laser2. The second case is an 18-year-old woman who had symptoms of fever, hepatomegaly, and weight loss. Through investigation, a mass was found in the right lobe of the liver, that was initially treated as an abscess, and upon the persistently non-improving picture, she underwent a biopsy, with a diagnosis of HCC. This patient died 4 days after the biopsy, with a severe clinical status1. The third case relates to a 34-year-old male patient CFLD, who was diagnosed with HCC after a biopsy of a lesion initially found during an annual abdominal ultrasound. The characteristic feature of this patient is that he had a normal α-fetoprotein (αFP) value with an increase of Ca19-9. He did not meet the liver transplant criteria, as his mass was of 7 cm and sorafenib was initiated, just like for our own patient3.
Additionally, what is of interest in our patient is that the diagnosis was performed on the basis of the imaging findings and the extremely high value of α-fetoprotein (665.73 ng/mL), as values greater than 400–500 ng/mL are considered diagnostic for HCC, without performing a liver biopsy6,8.
CONCLUSION
This is an uncommon case of hepatocellular carcinoma in a patient with cystic fibrosis. The annual examination led to an unexpected finding, which highlights the need for more regular screening of patients with cystic fibrosis and hepatic insufficiency, perhaps twice a year, with an abdominal ultrasound and possibly measuring α-fetoprotein, for early diagnosis of an eventual malignancy. As the survival of patients with cystic fibrosis grows, so do previously unknown manifestations and complications of the disease, involving a wide range of medical specialties.