INTRODUCTION
Pulmonary alveolar proteinosis (PAP) is a rare syndrome that causes hypoxemic respiratory failure characterized by the accumulation of lipoproteinous material in the alveoli1-3. The estimated prevalence and incidence have been reported as 3.7–6.9 per million and less than 1 per million in various studies, respectively4. According to the etiology, it is divided into three main categories; primary (idiopathic or autoimmune), secondary, or genetic. Anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies play a role in autoimmune PAP, while toxic inhalation or hematological disorders play a role in secondary PAP5.
In recent years, although new treatments such as inhaled GM-CSF3,6, plasmapheresis7, and rituximab8 have been on the agenda, whole lung lavage (WLL) which can provide long-term benefits in most patients, still maintains its place as the standard treatment7. The therapeutic effectiveness of WLL is achieved by physically removing the material accumulated in the alveoli with saline fluid. The procedure is often performed under general anesthesia and a double-lumen intubation tube is used. While one lung is mechanically ventilated, the opposite lung is lavaged. First, the alveoli are filled with saline fluid and the fluid is drained using the effect of gravity. The same process is repeated until the fluid drained from the alveoli is clear9. WLL procedures are still performed according to the first description by Ramirez-Rivera1 in 1963. However, several centers have made modifications to improve this original method10. However, there is no standardization among centers regarding specific indications and outcome criteria. The procedure still shows significant variability between centers11. Training on how to apply WLL is often based on a master-apprentice relationship or is self-taught. This is another reason for the differences in the technique between centers9.
In an article evaluating the WLL results of the 1110 procedures, the manageable complication rate was reported as 16% and the mortality rate as 1.1%. The most common complication after the procedure is fever9. Studies are still needed to optimize and standardize the procedure. There are few studies on this subject11. This study aimed to evaluate the technical aspects of WLL procedures performed in our bronchology unit, complications after the procedure, and factors affecting the complications.
METHODS
Ethics
Written informed consent was obtained from each patient. The study protocol was approved by the Ethics Committee of the University of Health Sciences Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital and was conducted under the Declaration of Helsinki.
Patient selection
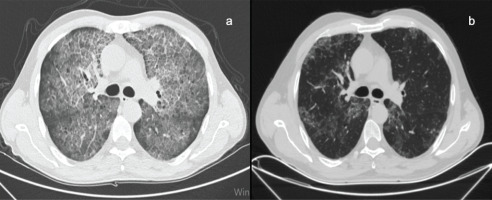
A total of 83 WLL procedures performed in our interventional bronchology unit between April 2010 and April 2020 were reviewed retrospectively. Patients with characteristic chest high-resolution computed tomography (HRCT) findings for PAP (Figure 1a) and histopathologically diagnosed by bronchoalveolar lavage or biopsy were included in the study. Patients aged <18 years were excluded from the study. The information of all patients was obtained from the medical records of our hospital.
Figure 1
a) HRCT scan axial slice is seen of the patient with PAP, b) HRCT scan axial slice of the same patient 2 months after bilateral WLL
The decision to perform WLL in our clinic was made according to the following criteria defined by Michaud et al.12. The procedure was planned, if any of these criteria are met:
PaO2 at rest <65 mmHg;
PA-aO2 >40 mmHg;
Worsening of symptoms or progressive respiratory failure in the patient follow-up (>10% decrease in PaO2 or need for O2 therapy or desaturation with exercise); and
Worsening of previous findings on HRCT or formation of new infiltrates characteristic of PAP procedure.
In patients who will undergo WLL for the first time, the procedure is primarily performed on the left lung, due to its relatively smaller size. In the second session, it was planned to be applied to the right lung. In patients who had a previous history of WLL, the procedure was performed on whichever side had more involvement according to HRCT findings (Figure 1b).
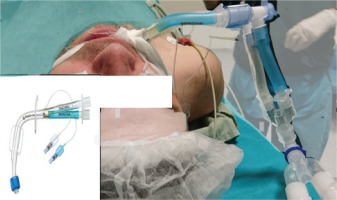
After obtaining written consent from the patients, WLL was performed in the operating room under general anesthesia via rigid bronchoscopy (Karl Storz Instruments, Germany). General anesthesia consisted of total intravenous anesthesia and the patients were ventilated with an air-oxygen mixture. After adequate muscle relaxation is achieved, selective intubation is provided with a double-lumen tube (Figure 2).
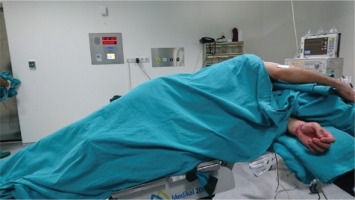
Confirmation that the intubation tube is in the proper position is verified by entering from both lumens with a thin fiberoptic bronchoscope. The patient is fixed by placing a lateral position at an angle of 90°, with the side to be washed on top. The location of the tube was checked again with a thin fiberoptic bronchoscope. The intubation tube channel ventilating the target lung was closed with a medical clamp. After the patient is placed in the reverse Trendelenburg position, saline warmed to 37℃ is infused through the intubation tube channel leading to the target lung (Figure 3).
After one liter (L) of saline is given to the lung, the patient is placed in the Trendelenburg position and free drainage of the fluid is ensured by the effect of gravity. During this drainage, manual percussion is applied to the patient without any assistive device. The same process is repeated until the drained fluid becomes clear (Figure 4).
The fluid deficit after each infusion and drainage is recorded. If the fluid deficit is ≥1000 mL during the procedure, intravenous diuretics are administered. After WLL is completed, both lungs are ventilated and the patient is placed in the supine position. Neuromuscular blockade is removed with 2–4 mg/kg sugammadex. Patients with adequate spontaneous respiratory effort and adequate cough reflex are extubated. All patients are followed up in the intensive care unit for at least 12 hours after the procedure13.
Statistical analysis
SPSS for Mac Version 20.00 (SPSS Inc., Chicago, IL., USA) package program was used in the statistical analysis of the obtained data. Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as frequencies and percentages. The Kolmogorov-Smirnov test was used to evaluate whether the continuous variables showed a normal distribution. The chi-squared test for categorical variables, ANOVA for continuous variables, and post hoc Tukey HSD were used to compare groups. A p<0.05 was taken as statistical significance.
RESULTS
A total of 83 WLL procedures were performed over 10 years in our unit on 21 patients (11 women), 42 of which were on the right and 41 on the left lung. Twenty of the PAP patients were autoimmune and 1 was secondary to hairy cell leukemia. The mean age of the patients was 38.2 ± 9.8 years. The most common complaints at the time of admission were shortness of breath (62.7%) and cough (18.1%). Of the patients, 6 (28.6%) were smokers, 6 (28.6%) were ex-smokers, and 9 (42.9%) were non-smokers. The mean duration of smoking among smokers was 14.9 ± 9.1 pack-years. There was no patient with a comorbidity. The general characteristics of the patients are summarized in Table 1.
Table 1
Clinical characteristics of patients who had WLL procedures
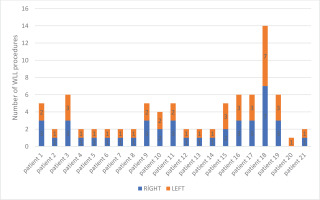
There was only one patient who underwent unilateral WLL only once because he did not come to follow-up sessions after the procedure. Right and left WLLs were performed on 10 patients once in separate sessions. The median number of procedures per patient was 2 (range: 1–14) (Figure 5).
The mean amount of fluid delivered to the airways during the procedures was 11.8 ± 4.3 L, and the difference (fluid gap) between the injected and free-drained fluid was a median of 550 mL (range: 0–4300). After the procedure, 9 (10.8%) complications were seen in 6 patients. Oxygen desaturation, which was described as SpO2 <90%, was observed during the procedure in 3 patients. In one of these three patients, oxygen desaturation did not require termination of the procedure. However, one patient also developed arrhythmia and the procedure was terminated, with the patient followed intubated in the intensive care unit and extubated on the 5th day. The fluid deficit of this patient after the procedure was 4300 mL. The other patient, whose procedure was terminated, was followed with nasal oxygen.
One patient had an epileptic seizure secondary to intracranial hemorrhage without sequelae on the 3rd day of follow-up. Short-term bronchospasm was observed in 1 patient after extubation and was followed in the intensive care unit for 1 day after the procedure. Klebsiella pneumonia developed in one patient within the first week after the procedure. The same patient also had transient neuropathy secondary to brachial plexus compression due to lateral position, which improved with physiotherapy. No patient died during the procedure and in the first 30 days after the procedure. While oxygen support by nasal cannula or mask was required after 59 (71.1%) procedures, NIMV (non-invasive mechanical ventilation) support was required after 17 (20.5%) procedures and IMV support was required after 7 (8.4%) procedures. The median need for IMV was 7 (5–120) hours. The characteristics of the procedures performed are given in Table 2. There was no significant relationship between the mechanical ventilation needs of the patients and their age (p=0.470). While the mean fluid deficit of the patients who needed oxygen support was 485.1 ± 387 mL, it was 646.1 ± 652 mL for those who needed NIMV support, and 1400 ± 1419 mL for those who needed IMV support, and there was a statistically significant difference between the groups in terms of fluid deficit (p<0.001) (Table 3).
Table 2
Characteristics and complications of WLL (N=83)
| Characteristics | n (%) |
|---|---|
| Number of WLL per patient, median (range) | 2 (1–14) |
| Fluid details | |
| Amount of fluid delivered to the lung (L), mean ± SD (range) | 11.8 ± 4.3 (1–23) |
| Amount of free-draining fluid from the lung (L), mean ± SD | 11.3 ± 4.5 |
| Fluid gap (mL), median (range) | 550 (0–4300) |
| Complications* | |
| Desaturation | 3 (3.6) |
| Bronchospasm | 1 (1.2) |
| Arrhythmia | 1 (1.2) |
| Pneumonia | 1 (1.2) |
| Epileptic seizure (secondary to ICH) | 1 (1.2) |
| Transient neuropathy | 1 (1.2) |
| Prolonged intubation** | 1 (1.2) |
| Total | 9 (10.8) |
| Post-procedure status of the patient | |
| Extubated, needed O2 support | 59 (71.1) |
| Extubated, needed NIMV support | 17 (20.5) |
| İntubated | 7 (8.4) |
Table 3
Need for mechanical ventilation increases as the fluid gap increases
| Oxygen support | NIMV | IMV | p | |
|---|---|---|---|---|
| Fluid gap (mL), mean ± SD | 485 ± 387 | 646 ± 652 | 1400 ± 1419 | <0.001 |
| Age (years), mean ± SD | 37 ± 9 | 36 ± 7 | 33 ± 5 | 0.470 |
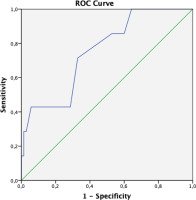
ROC analysis was performed to determine the cut-off value for the fluid gap in predicting the patient’s intubation risk after the procedure. When the cut-off value for the fluid gap was taken as 550 mL, its sensitivity in predicting the intubation of the patient was 71.4%, and its specificity was 67.1% (Figure 6).
DISCUSSION
Since PAP is a rare disease, WLL is performed in a few centers worldwide. Therefore, data on the WLL procedure are limited. Also, the experience of WLL in a limited number of centers in our country is limited to case reports. In this study, where we share our experiences in the last 10 years, the data of 83 WLL were presented. To the best of our knowledge, this is the first study to show a linear relationship between fluid gap during the procedure and the need for mechanical ventilation after the procedure.
When the limited number of studies are evaluated, it is seen that the WLL procedure still has not gained standardization, and different techniques are used, from positioning the patient during the procedure to the amount of fluid used, and the preference for single or double lung lavage. Regarding positioning in published studies, it is reported that placing in the full lateral position can reduce the possibility of fluid leakage into the ventilated lung, whereas placing in the supine position throughout the procedure provides the opportunity to perform bilateral lung lavage in the same session and reduces the risk of endotracheal tube displacement14. We preferred a lateral position with an angle of 90° degrees in our procedures. When there was a suspicion of endotracheal tube displacement during lavage, control was achieved with a thin fiberoptic bronchoscope. Therefore, no displacement of the endotracheal tube or any related complication was observed. However, as a disadvantage of full lateral positioning, transient neuropathy due to brachial plexus compression was observed after one of the procedures in our patient who underwent 14 procedures.
WLL to each lung in different sessions is the generally accepted method. In a survey study involving 20 centers, it was reported that 17 (85%) of the centers preferred single lung lavage. In the same study, one center performed bilateral WLL in a single session, while 2 centers made a decision based on patient tolerance9. We also always do a single lung lavage as it is more reliable and tolerable. Abdelmalak et al.14, in their article reporting their experiences, state that hypotension develops requiring vasopressor, especially during the second lung wash of bilateral WLL. In the study of Silva et al.15, who preferred bilateral lung lavage, it was observed that patients often needed long-term mechanical ventilation. Most of the patients were extubated at least 18 hours after the procedure. There was no hypotension in any of our procedures. Non-invasive monitoring was sufficient for the blood pressure monitoring of all patients. In our cases who were intubated after the procedure, the median intubation time was 7 hours. After only one procedure, mechanical ventilation was needed for up to 120 hours. In this procedure, the patient had arrhythmia and desaturation. The post-procedural fluid deficit was 4300 mL. It was thought that these complications might be related to the excessive fluid deficit. When the relationship between fluid deficit and the outcome of our procedures was evaluated, it was seen that the need for mechanical ventilation increased as the fluid deficit increased, supporting our opinion. These results suggest that single lung lavage may be safer in each session.
Whole lung lavage is reported as a safe and effective method. In a multicenter survey study, the complication rate was reported as 18%. Fever, desaturation, bronchospasm, pneumonia, fluid leakage into the ventilated lung, pleural effusion, pneumothorax, and cardiac arrest are complications seen in order of decreasing frequency9. In another study, complications were seen in 11 of 33 patients (33%), and desaturation during the procedure was reported most frequently in 12% of the patients. Other complications are headache/fever (3%), subcutaneous emphysema (3%), pneumonia (3%), pulmonary edema (3%), intubation tube dislocation (3%), and death (6%)11. In the Zhao et al.16 study, the data of 80 patients were presented and no complication related to the procedure was reported after WLL. In our study, no death was observed in any of our patients during and in the first 30 days of the procedure, supporting that WLL is safe. Also, our procedural complication rate was 10.8%. When the literature about the complications of WLL was reviewed, no data were reported on intracranial hemorrhage. This complication was attributed to prophylactic low molecular heparin usage in the follow-up period. Similarly, no neuropathy secondary to brachial plexus compression was reported. It was attributed to the long procedure time performed in the lateral decubitus position. Klebsiella pneumonia was seen in one of our patients and its incidence was similar to the literature. These complications were all treated successfully without any sequelae. In addition, fever, pleural effusion, and pulmonary edema were not observed. In the Zhao et al.16 study, in which no complications were reported, there was no data on the total amount of fluid used during the procedure. In another study, procedures were performed with a total fluid of up to 40 L, and pulmonary edema and pleurisy were observed in this study. The authors reported that although the origin of edema is still not clear, it may be non-cardiogenic due to cardiac load or local inflammation11. The reason why we never encountered pulmonary edema was that the average amount of fluid used was 11.8 L, and it was thought to be related to its relatively low amount compared to the reported studies. In addition, it can be thought that close monitoring of fluid deficit during the procedure and administering diuretics to patients with a fluid deficit above 1000 mL may be effective in preventing pulmonary edema.
Hydropneumothorax and subcutaneous emphysema reported in some publications9 did not occur in any of our 83 procedures. We think that the absence of this complication is related to the experience of the anesthesiologist. Our hospital is a tertiary care center with an average of 250 procedures per year in the interventional bronchology unit.
No intubation tube dislocation was observed in our cases, as the location of the intubation tube was checked with a thin fiberoptic bronchoscope after the lateral positioning during WLL, and repeated checks were performed immediately in case of any doubt. Our desaturation rates were also lower than the literature. It was thought that the control of the airway when necessary with a thin fiberoptic bronchoscope during the procedure, the fact that the total amount of fluid given is relatively low compared to the literature, and the preference for single lung lavage in each session in all our patients, may be effective in the low incidence of desaturation.
WLL was applied safely and successfully in all patients. Inhaled GM-CSF was given to 4 patients with the highest number of WLL with successful results, which should be considered as an alternative treatment for PAP patients17-19.
Limitations
There are some limitations to our study. Due to the retrospective nature of our study, there were no records of whether the arterial blood gases of each patient were taken at room air. Therefore, a comparison of ABG before and after treatment, which is the most important indicator of treatment response, could not be made. Whether pre-procedural ABG parameters were effective in determining the need for post-procedural mechanical ventilation could not be evaluated. Although DLCO results of patients in their stable periods were available, this parameter was excluded from the evaluation since the WLL was performed in case of respiratory failure and most of them could not cooperate with DLCO. It could be predicted that the number of admissions to hospitals may have decreased due to the concerns of the patients and that the number of procedures may have decreased due to short-term restrictions in the use of operation rooms as per the precautions taken throughout the country in the first peak period of the COVID-19 pandemic.
CONCLUSIONS
This study shares the experience of one of the centers that perform the largest number of whole lung lavages. According to our experience performing a single lung lavage in each session may be more reliable, and using a thin fiberoptic bronchoscope could help prevent endotracheal tube dislocation. The need for mechanical ventilation following the procedure has been seen in patients with a high fluid deficit, and it can help predict patients who may need ventilation at the end of the procedure. Being cautious from the beginning and providing intensive care conditions for the patient helps us to foresee outcomes.