INTRODUCTION
Asthma is an inflammatory disorder defined by variability of respiratory symptoms and airflow limitation. Asthma control is assessed by symptom control and future risk of adverse outcomes and remains a challenge for clinicians, especially for patients with difficult-to-treat or severe asthma, i.e. uncontrolled on medium or high doses of inhaled corticosteroids/long-acting β2-agonist (ICS-LABA). In such patients, information regarding daily disease status may improve patient-physician dialogue and symptom control1.
Among the parameters used for asthma monitoring, Peak Expiratory Flow (PEF) has been widely used in the past, especially for early detection of asthma exacerbations and assessment of treatment response. Traditionally, patients record symptoms and PEF in a paper diary, which they use to adjust therapy in accordance with written asthma plans. The use of digital technology and telemedicine in the monitoring of patients with asthma is rapidly increasing. Telemonitoring augments self-monitoring and disease management by facilitating patients to record their level of asthma control electronically (e.g. on a smartphone), promptly alerting them about deteriorating control, and supporting remote physician consultations.
In this study, we evaluated the quality of home-based spirometry in patients with asthma using the NuvoAir platform (NuvoAir U.S. inc, Boston, M.A., U.S.A), which consists of a smartphone application, a Bluetooth spirometer (AirNext), and physician portal. The software in the application provides feedback to patients regarding spirometry quality, coaching patients to improve their spirometry technique.
METHODS
Consecutive adults with difficult-to-treat or severe asthma were trained on the AirNext spirometer and were asked to perform home spirometry daily for the first month and then weekly or on symptom worsening. Geolocation data were used to identify patients’ first spirometry session in the hospital (‘supervised’) and sessions performed at home (‘unsupervised’). All patients provided informed consent.
These analyses used data from patients who performed their first unsupervised spirometry within 30 days of their supervised spirometry. The grading of the quality of spirometry sessions was based on the American Thoracic Society Technical Statement2; briefly spirometry tests are graded either manually or by a spirometry software with grades A–F. Spirometry sessions with grades A–C are usable, while grade D tests are suspect. Sessions with grade E can be used only to show values within the normal range, while tests with grade F are not usable. Each test is graded according to the acceptability and repeatability criteria. A spirometry session must have at least two acceptable tests with repeatability within 0.200 L to be considered usable. An acceptable test must be free from artifacts, without coughing during the first second (for FEV1), or early termination (for FVC). Acceptability criteria also include a good start of exhalation, with extrapolated volume <5% of FVC or 0.150 L, maximal effort throughout the maneuver, no obstructed mouthpiece, no glottis closure or abrupt termination. In this study data are presented as descriptive statistics (proportions and/or mean ± standard deviation).
RESULTS
A total of 10 asthma patients (four uncontrolled; six partly controlled [GINA 2018]) were included, 9 (90%) female, mean age 42.0 ± 10.7 years, with FEV1 69.6 ± 26.2% (range: 27.3–101.0) predicted at the supervised session. Patients performed 1235 unsupervised sessions over 181.5 ± 153.9 days (maximum 382 days), of which 877 (71.0%) were of acceptable quality (grades A–C, at least two maneuvers, <200 mL FEV1 and FVC variation), the majority of these (556) being grade A (Figure 1A). In comparison, 50.0% of the 10 supervised sessions were acceptable (Figure 1A).
Figure 1
A) Session grading in supervised and unsupervised sessions; B) Spirometry results from supervised and unsupervised sessions
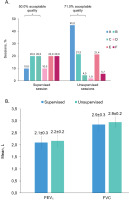
Spirometry results were similar when comparing the supervised assessment at hospital with the first unsupervised assessment at home (Figure 1B). The values for FEV1 and FVC were 2.1 ± 0.3 L and 2.9 ± 0.3 L, respectively, from the supervised sessions, while at the first unsupervised home assessment they were 2.2 ± 0.2 L and 2.9 ± 0.2 L, respectively. Additionally, the mean FEV1 predicted values were 69.6 ± 26.2% and 72.0 ± 24.0%, respectively.
DISCUSSION
In this pilot study, we demonstrated that patients with asthma can perform unsupervised home spirometry of acceptable quality over prolonged periods of time, supporting self-management, and potentially optimizing patient care.
Asthma is an ideal target for digital oriented or home-based interventions, because it usually affects younger patients that are more familiar with digital technology3. Nowadays, more and more applications are designed for asthma patients to perform pulmonary function tests through mobile spirometers and record symptoms through questionnaires, giving them feedback on managing exacerbations and instructions for the proper use of inhalers, improving symptom control, and self-management4. Through these applications, clinicians can also have an overview of the patients’ status and advise accordingly5. Virtual care is shown as effective as in-person visits for outpatient treatment of asthma, improving asthma control, and quality of life6,7.
PEF is widely used as standard of care in asthma monitoring, especially in severe asthma, but it is effort-dependent (therefore its success may rely on subjective evaluation by the patients or their caretakers), whereas in full spirometry, the physician can evaluate the patient’s effort and assess more lung function parameters. Portable spirometry may reveal earlier subtle deterioration in lung function, long before clinically meaningful symptoms, allowing for earlier and more effective management.
During the COVID-19 pandemic, the avoidance of emergency department visits was encouraged and the performance of lung function tests was restricted. This led to an increased utilization of telemedicine consultation, and the use of peak flow meters and portable spirometry were widely reported8. Meanwhile, new diagnostic tools have emerged, using machine learning algorithms. These tools use data based on simple questions about patient symptoms and portable spirometry parameters and aid diagnosis, but this could be expanded to asthma monitoring9.
Two recent articles were published comparing unsupervised home spirometry and clinic spirometry, a meta-analysis by Anand et al.10 and a post hoc analysis by Oppenheimer et al.11, both concluding that home spirometry is less consistent than and lacked agreement with clinic spirometry, suggesting that unsupervised home readings are not interchangeable with clinic measurements. Nevertheless, the meta-analysis by Anand et al.10 included studies of all clinical conditions, with significant heterogeneity among outcomes, while we focused on asthma patients, who seem to be ideal candidates for such an intervention. Furthermore, different portable devices were used in the studies included in the meta-analysis and the study by Oppenheimer et al.11, thus the results may only apply to those specific spirometers.
Moreover, our patients were trained adequately on the AirNext spirometer before performing unsupervised tests, while the NuvoAir platform aids technique improvement by providing data on spirometry quality. Finally, despite the small sample size, there was a large number of spirometry tests performed at home, demonstrating acceptable quality.



