INTRODUCTION
Pleural effusion and hemoptysis may present due to multiple etiological factors, but patients with such clinical manifestations are mainly admitted to the pulmonary department for diagnosis and treatment1. Nevertheless, misdiagnosis is likely to happen if doctors are not aware of potential, though peculiar, etiological factors1-3, such as secondary to AF ablation pulmonary vein stenosis-PVS1,4.
From a multicenter systematic evaluation, PVS due to AF radiofrequency ablation has a reported incidence of 20.8%, and in the majority of cases, remains asymptomatic4. In the presented manuscript, we report a rare case of a man that underwent AF ablation 4 months before, presenting pleural effusion and hemoptysis.
The following case report, written according to the CARE checklist, describes in detail the patient’s management and clinical course, and aims to highlight the key role of pneumonologists’ clinical awareness, for the prompt diagnosis and treatment of PVS.
CASE PRESENTATION
A 43-year-old Caucasian male, smoker, without occupational exposure to inhaling substances, proceeded to our institution on August 2023, complaining about shortness of breath for the past week. The patient’s history included dyslipidemia and depression, treated with atorvastatin and escitalopram 20 mg, respectively. On March 2023, due to atrial fibrillation non-responding to anti-arrhythmic medications, the patient underwent radiofrequency AF ablation. The patient’s echocardiography at 3 months post-AF ablation was unremarkable.
The patient’s vital signs were: BP 140/90 mmHg, PR 109 beats/minute, SpO2 89%, and a body temperature of 36.8℃. Clinical examination revealed breath sounds’ absence at the right base and dullness to percussion. Subsequent CXR verified right pleural effusion. After a Bülau drainage insertion, 2 L of polymorphonuclear exudate was drained. Pleural fluid cytology was negative for cancer cells. Blood tests revealed increased inflammatory markers (CRP 105 mg/L and mild neutrophilia). The patient was immediately administrated with IV moxifloxacin and admitted for further investigation and treatment.
Upon admission, the patient underwent blood test also for: troponins, CK-MB, antinuclear antibodies, rheumatic factor, native-DNA antibody and anti-extractable nuclear antigen analyses, viral infections (such as SARS-CoV-2, HIV, HBV, HCV, CMV, and EBV), blood cultures collected during fever peaks for infectious diseases (leptospirosis, borreliosis, brucellosis, rickettsiosis), and tumor markers. All the laboratory results were negative. Tuberculin skin testing (TST), blood, and urine sample culture tests were also negative and D-dimers were 6.85 μg/mL.
Subsequent HRCT indicated patchy infiltrates of the right pulmonary basis and the CTPA performed due to elevated D-dimers revealed minor filling defects of segmental vessel branches of the right pulmonary artery, without lesions of pulmonary veins. The patient was treated with antibiotics for the pneumonia, and in agreement with the cardiologists, he was finally discharged the 7th day with rivaroxaban 20 mg od.
After a month, on September 2023, the patient proceeded once again to our institution complaining about a sole incidence of hemoptysis (<10 cc) and body temperature 37.6℃ for the last 12 hours. Blood tests, urine analysis and blood cultures were unremarkable. CXR did not indicate pleural effusion.
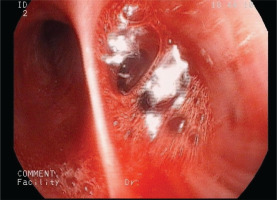
Following these, a bronchoalveolar lavage (BAL) was performed. The bronchial tree anatomy was normal. However, physicians discovered bleeding of RB9 and RB10 (Figure 1). The diagnosis of hemorrhage was confirmed since counting 57% hemosiderin-laden macrophages in BAL fluid. The BAL fluid was negative for common pathogens, Ziehl-Neelsen stain and mucus.
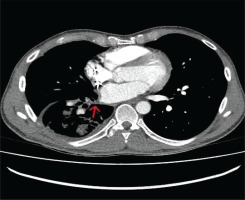
Patient remained asymptomatic and afebrile during admission and treated with piperacillin/tazobactam. The 5th day of hospitalization, he underwent CTA of thoracic aorta and CTV of thoracic veins. The exam revealed severe pulmonary vein stenosis, almost occlusion, which was considered to be a complication of radiofrequency AF ablation due to the patient’s history (Figure 2). Afterwards, the patient was admitted to the cardiothoracic surgery department, of another surgical institution where he was scheduled for lobectomy due to his recurrent symptoms of respiratory infections and hemoptysis. When the patient was discharged, the cardiologic medication remained intact.
In a follow-up appointment after a month, the patient presented with no complications or respiratory symptoms. Physical examination, CXR, blood tests and vital signs were within normal range. Echocardiography was unremarkable.
DISCUSSION
PVS constitutes a rare clinical condition that may be acquired or congenital1,2. Congenital PVS is typically presented in childhood, though acquired PVS presents in adults, as a complication of catheter ablation interventions for arrhythmias, including radiofrequency AF ablation1,2,5-7, rare cases, such as mediastinal tumors8 and cardiac surgery7. From the largest multicenter systematic evaluation, PVS due to AF radiofrequency ablation has an incidence of 20.8%4.
Although the exact underlying molecular mechanism of PVS remains vague, it is reported that scarring of the pulmonary venous wall, the contraction and the peri-adventitial inflammation of the vein due to the thermal injury provoked by AF ablation, may narrow or even occlude the lumen of the pulmonary vein2,4.
There are documented predictors of PVS due to AF ablation, such as previous AF ablation and diabetes1,4. More specifically, it is reported that diabetes is associated with both structural and electrical remodeling, leading to microvascular and macrovascular complications4.
In the majority of cases, PVS due to AF ablation is mild or moderate, and remains asymptomatic4. The clinical manifestations of PVS vary depending on: 1) the number of pulmonary veins involved, 2) the severity of the stenosis, 3) the response of the pulmonary vasculature to the stenosis, 4) the time course of PVS, 5) the patient’s clinical status, and 6) the presence or not of collateral vessels2. Patients may present chest pain, dyspnea, orthopnea, cough, pneumonia, recurrent pulmonary infections, and more rarely hemoptysis, as in the presented case1,2,4.
The incidence of post-ablation PVS may lead to major hemodynamic changes, such as pulmonary hypertension and subsequent complete occlusion of the pulmonary vein4. These changes are responsible of the vulnerability to recurrent or/and drug-resistant pulmonary infections, that may present with pleural effusion, as in the presented case4,5,7. Hemoptysis, constitutes an infrequent manifestation of PVS, which is related to the increased venous pressure in the pre-stenotic zone and the subsequent lung tissue congestion and bleeding2.
The diagnosis of PVS is not easily identified, given its rarity, and the variability of its clinical manifestations and non-specific radiological findings1,4-7. Differential diagnosis includes other causes of pleural effusion (such as cancer, thoracic mass, and pneumonia), pulmonary embolism1,3, NOACs, and hemorrhagic pulmonary vasculitis2. Common radiological exams, such as CXR and thoracic CT typically demonstrate irrelevant to PVS features1,2,5.
Herein, CTPA and CTA are of paramount significance when encountering a patient with hemoptysis and previous AF ablation2,4,5,7. Indeed, CTA may detect the exact location and extent of probable pulmonary vein stenosis. In addition, following CT-scans, bronchoscopy plays a key role in the differential diagnosis of PVS, especially when encountering a patient with hemoptysis, as in the presented case2. Finally, congenital PVS was excluded from the differential diagnosis, since the patient remained asymptomatic through childhood and adulthood, until performing AF ablation.
Post-ablation PVS may be treated via balloon dilatation of the pulmonary vein; however, re-stenosis within 1 year constitutes a common complication, with an incidence of approximately 50%2. Therefore, patients with PVS should have regular follow-up and echocardiography1,2. In addition, in cases of recurrent episodes of pulmonary infections or respiratory failure or bleeding, as in the presented case, lobectomy should be considered as a therapeutic solution5,9. However, the best therapeutic approach is the prevention of PVS1,4. This strategy is mostly related to reduction of ablation’s temperature and energy, and placing the ablation site from inside to outside the orifice of the pulmonary vein2.
CONCLUSION
Physicians’ clinical awareness is of paramount significance when encountering a patient with pulmonary infection and hemoptysis, who underwent catheter ablation. The presence of pleural effusion and hemoptysis after AF radiofrequency ablation should raise the suspicion of PVS and a CTA should be immediately performed for the prompt diagnosis and the adequate treatment of PVS.